GROUP LEADER
Antonio Marchetti
Antonio Marchetti is group leader at the CAST Institute since its foundation in 2019. His group has a large experience in molecular pathology at both clinical an experimental level. His research activity is presently conducted in 4 main branches of interest: oncogenomics, molecular diagnostics in oncology, experimental pathology, and digital-quantitative pathology.
Oncogenomic branch
This branch of interest is mainly dedicated to the development of innovative technical approaches and strategies for molecular characterization of solid tumors.
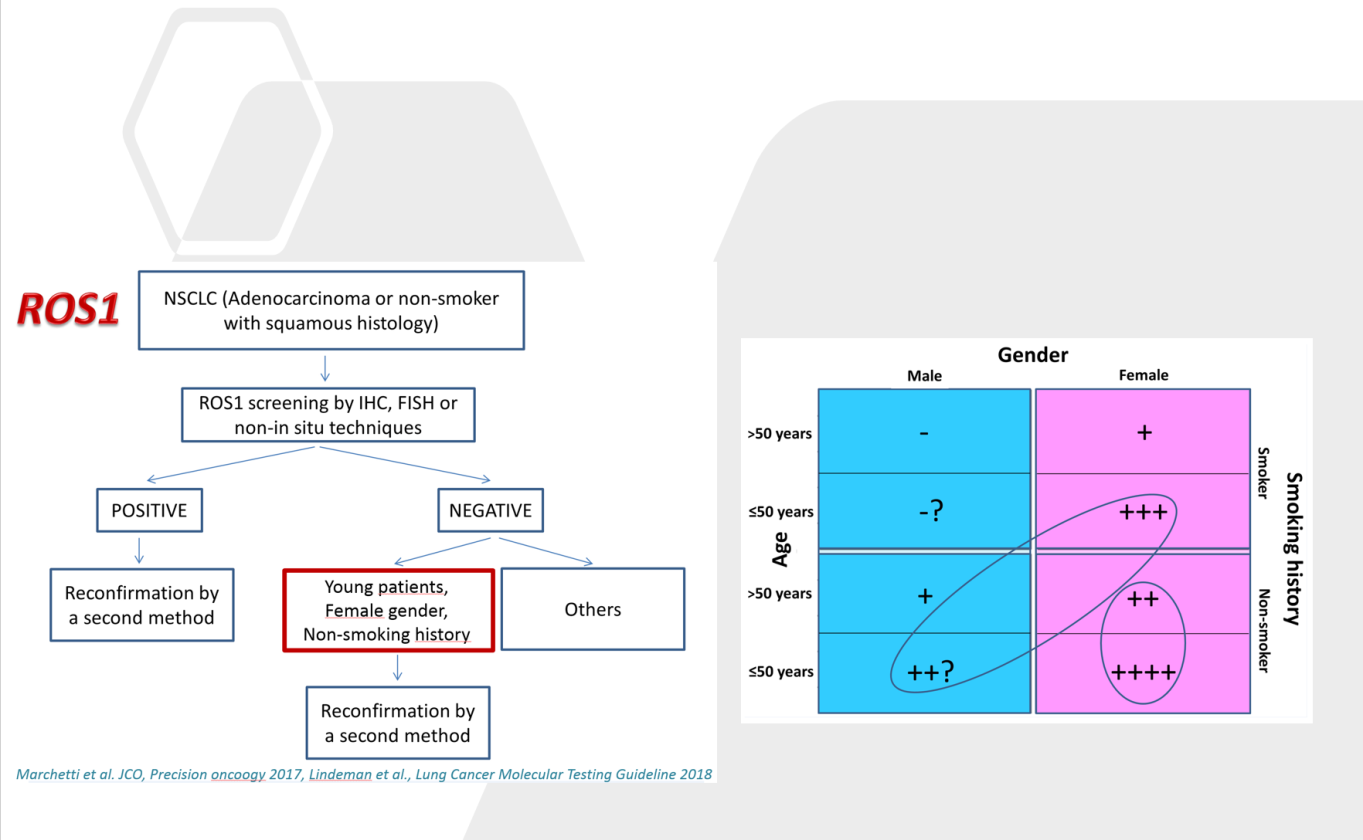
At present the group is particularly focused on the analysis of rare molecular alterations characterizing tumors developed in young patients. New drugs for precision medicine are showing impressive activity in individuals carrying such rare mutations, including a number of genetic fusions.
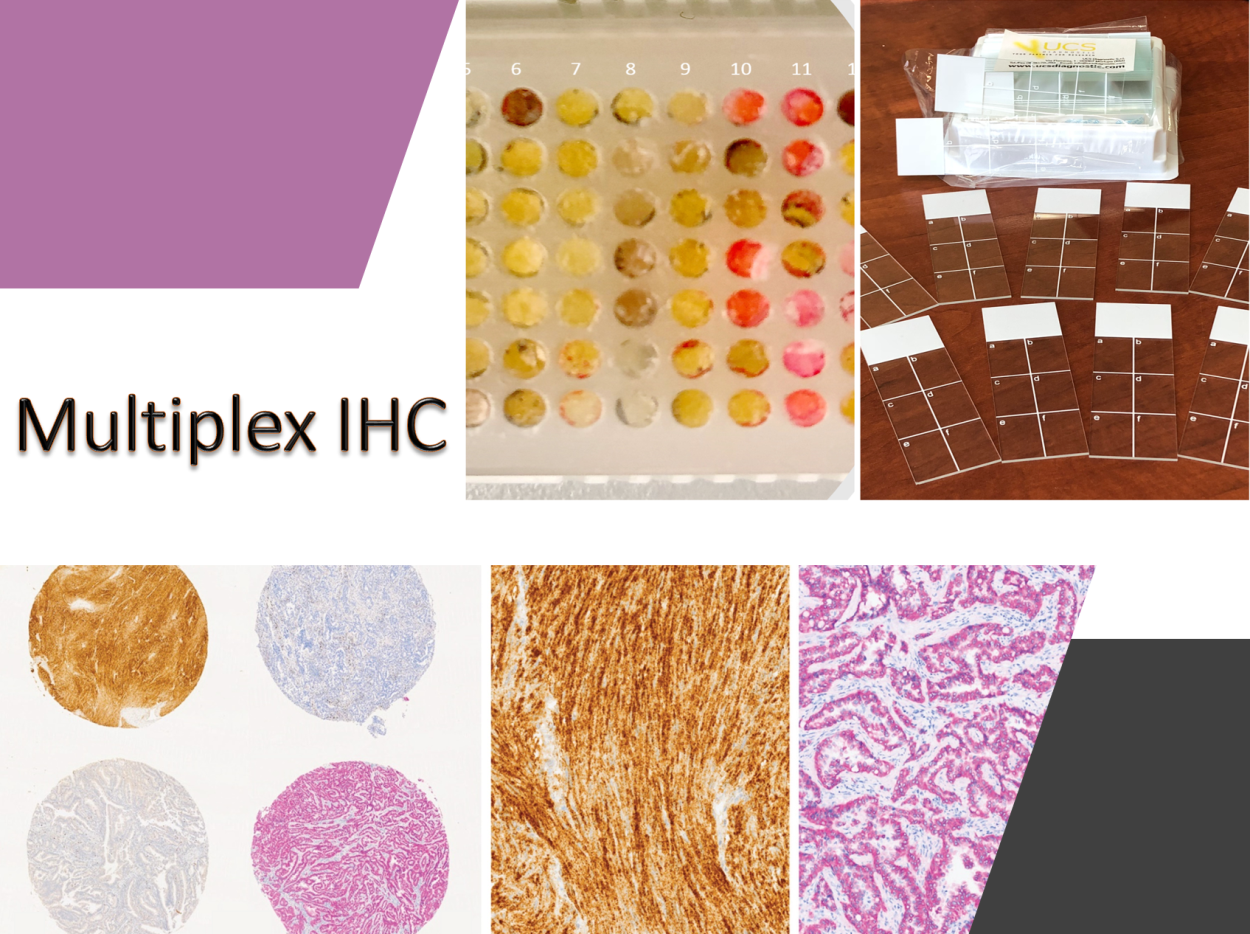
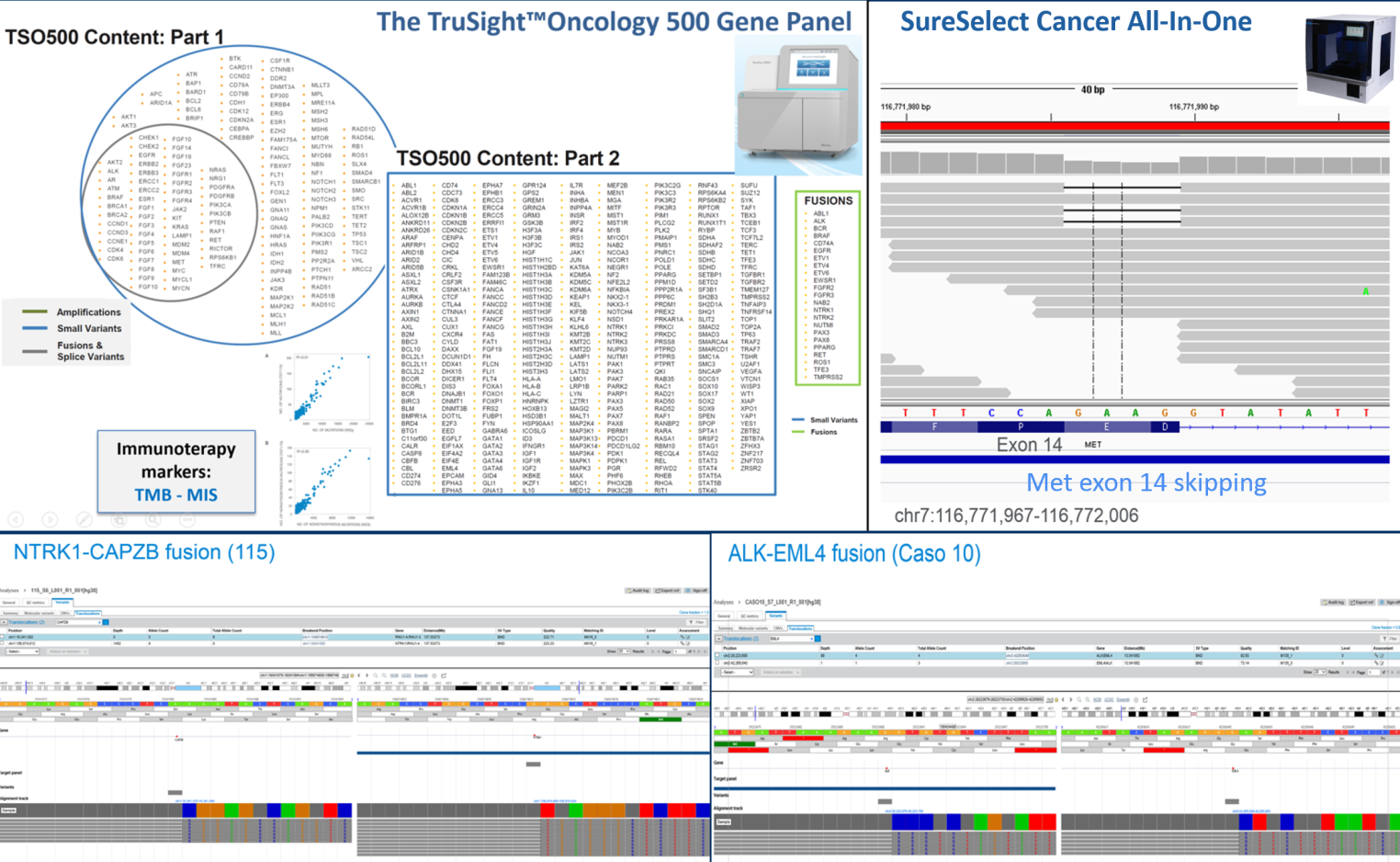
Nevertheless, few data exist on the incidence of these genomic events in solid tumors and few patients are currently tested in clinical practice. Recent data reported by the group using different technical approaches suggest a higher incidence of genetic fusions in tumors from young patients (under 50 years old). The investigation of rare mutations requires the analysis of large series of patients with sensitive and affordable molecular biology techniques including, multiplexing immunohistochemistry, mutation-oriented quantitative RT-PCR, Fluorescence in situ hybridization (FISH) and a number of next generation sequencing (NGS) strategies. In this latter area the group has a decennial experience, been one of the first lab in Italy that introduced next generation sequencing in histopathology and cytopathology.
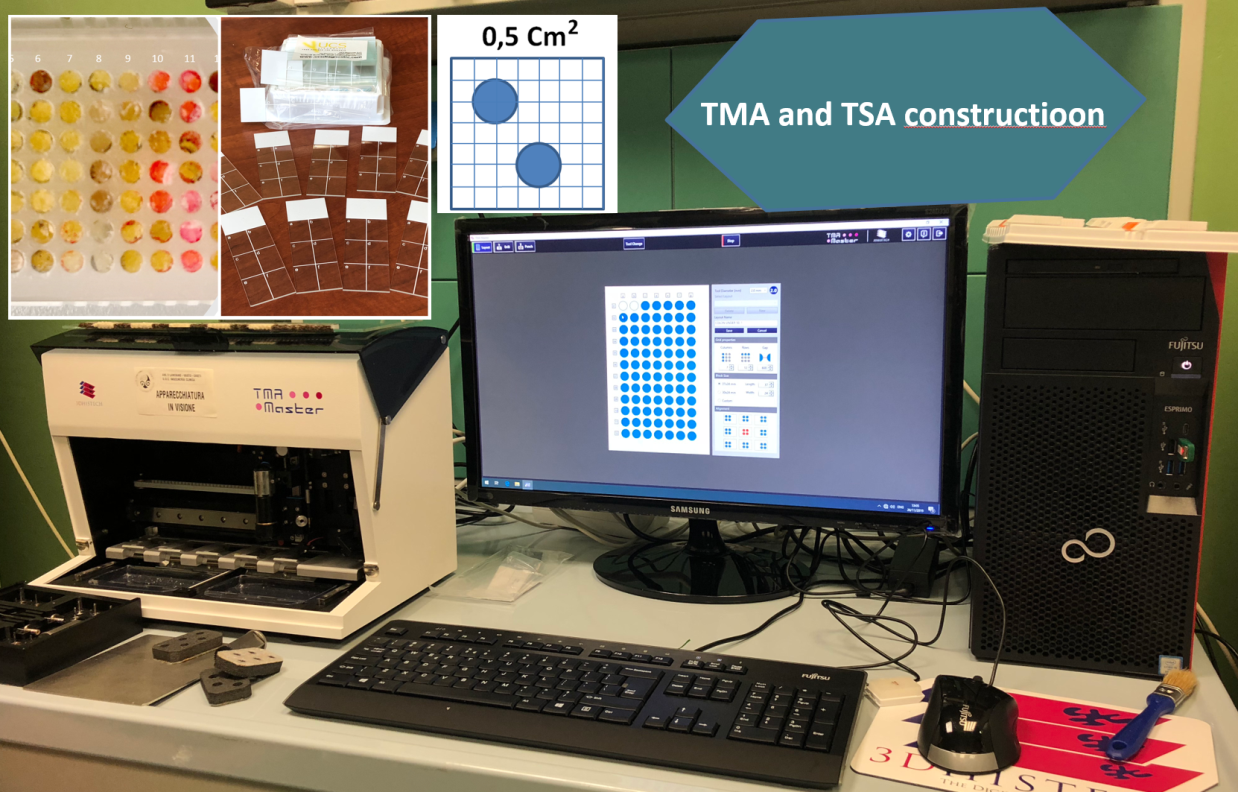
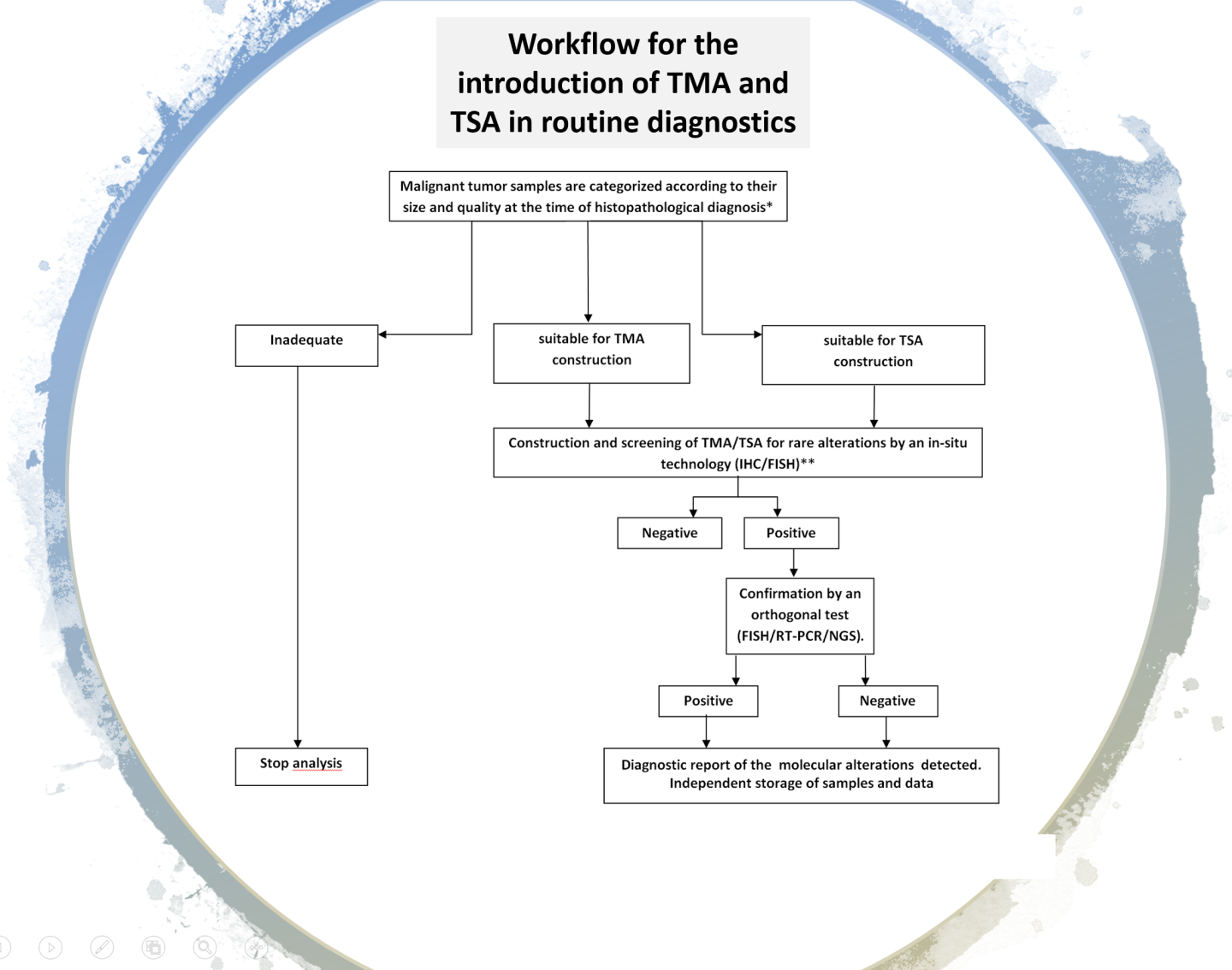
Large scale screening with conventional immunohistochemical approaches, followed by orthogonal confirmation tests, has been suggested as a potential innovative diagnostic strategy for the detection of rare mutational events. However, if the test must be extended to all patients with solid tumors it has unacceptable costs per treatable patient in clinical practice and is time consuming. An efficient, rapid and economically affordable new pathological screening strategy for the detection of rare molecular targets to select cancer patients for histology-agnostic treatments has recently been developed (Marchetti et al., 2019). The strategy is based on the construction of Tissue MicroArrays (TMA) for high throughput analysis of selected tumor areas from each patient. The application of this new strategy in a multicenter retrospective/(prospective) study will allow to get information about the incidence and distribution of these rare gene fusions in a large population of young patients with malignant solid tumors. A large National study under the patronage of the Italian Society of Pathology, involving 19 referral oncologic centers, coordinated by Prof. Antonio Marchetti has recently been activated. The diagnostic strategy will be applied to a large series of 12.000 oncological patients under 50 years old and will be based on a IHC screening on multi-samples followed by confirmation with orthogonal methods (NGS, FISH) in positive or equivocal cases.
In the last five years the group has developed and applied a number of other different technical approaches for the detection of rare mutations and fusions by RT-PCR methods and NGS using both amplicon and hybridization-based technologies.
DETECTION OF COMPLEX GENOMIC ALTERATIONS BY NGS TECHNOLOGY
READ MORE
- Marchetti A, Di Lorito A, Felicioni L, Buttitta F. An innovative diagnostic strategy for the detection of rare molecular targets to select cancer patients for tumor-agnostic treatments. Oncotarget. 2019 Dec 10;10(65):6957-6968. doi: 10.18632/oncotarget.27343. PMID: 31857850; PMCID: PMC6916754.
- Paolini D, Tiseo M, Demma F, Furneri G, Dionisi M, Akkermans M, Marchetti A. Ventana ALK (D5F3) in the Detection of Patients Affected by Anaplastic Lymphoma Kinase-positive Non-Small-cell Lung Cancer: Clinical and Budget Effect. Clin Lung Cancer. 2018 Sep;19(5):e735-e743. doi: 10.1016/j.cllc.2018.05.012. Epub 2018 May 31. PMID: 29937385.
- Rulle U, Tsourti Z, Casanova R, Deml KF, Verbeken E, Thunnissen E, Warth A, Cheney R, Sejda A, Speel EJ, Madsen LB, Nonaka D, Navarro A, Sansano I, Marchetti A, Finn SP, Monkhorst K, Kerr KM, Haberecker M, Wu C, Zygoura P, Kammler R, Geiger T, Gendreau S, Schulze K, Vrugt B, Wild P, Moch H, Weder W, Ciftlik AT, Dafni U, Peters S, Bubendorf L, Stahel RA, Soltermann A. Computer- Based Intensity Measurement Assists Pathologists in Scoring Phosphatase and Tensin Homolog Immunohistochemistry - Clinical Associations in NSCLC Patients of the European Thoracic Oncology Platform Lungscape Cohort. J Thorac Oncol. 2018 Dec;13(12):1851-1863. doi: 10.1016/j.jtho.2018.08.2034. Epub 2018 Sep 18. PMID: 30240851.
- Letovanec I, Finn S, Zygoura P, Smyth P, Soltermann A, Bubendorf L, Speel EJ, Marchetti A, Nonaka D, Monkhorst K, Hager H, Martorell M, Sejda A, Cheney R, Hernandez-Losa J, Verbeken E, Weder W, Savic S, Di Lorito A, Navarro A, Felip E, Warth A, Baas P, Meldgaard P, Blackhall F, Dingemans AM, Dienemann H, Dziadziuszko R, Vansteenkiste J, O'Brien C, Geiger T, Sherlock J, Schageman J, Dafni U, Kammler R, Kerr K, Thunnissen E, Stahel R, Peters S; European Thoracic Oncology Platform Lungscape Consortium. Evaluation of NGS and RT-PCR Methods for ALK Rearrangement in European NSCLC Patients: Results from the European Thoracic Oncology Platform Lungscape Project. J Thorac Oncol. 2018 Mar;13(3):413-425. doi: 10.1016/j.jtho.2017.11.117. Epub 2017 Nov 27. PMID: 29191776.
- Marchetti A, Pace MV, Di Lorito A, Canarecci S, Felicioni L, D'Antuono T, Liberatore M, Filice G, Guetti L, Mucilli F, Buttitta F. Validation of a new algorithm for a quick and easy RT-PCR-based ALK test in a large series of lung adenocarcinomas: Comparison with FISH, immunohistochemistry and next generation sequencing assays. Lung Cancer. 2016 Sep;99:11-6. doi: 10.1016/j.lungcan.2016.06.005. Epub 2016 Jun 14. PMID: 27565907.
- Marchetti A, Palma JF, Felicioni L, De Pas TM, Chiari R, Del Grammastro M, Filice G, Ludovini V, Brandes AA, Chella A, Malorgio F, Guglielmi F, De Tursi M, Santoro A, Crinò L, Buttitta F. Early Prediction of Response to Tyrosine Kinase Inhibitors by Quantification of EGFR Mutations in Plasma of NSCLC Patients. J Thorac Oncol. 2015 Oct;10(10):1437-43. doi: 10.1097/JTO.0000000000000643. PMID: 26295376.
- Molinari F, Felicioni L, Buscarino M, De Dosso S, Buttitta F, Malatesta S, Movilia A, Luoni M, Boldorini R, Alabiso O, Girlando S, Soini B, Spitale A, Di Nicolantonio F, Saletti P, Crippa S, Mazzucchelli L, Marchetti A, Bardelli A, Frattini M. Increased detection sensitivity for KRAS mutations enhances the prediction of anti-EGFR monoclonal antibody resistance in metastatic colorectal cancer. Clin Cancer Res. 2011 Jul 15;17(14):4901-14. doi: 10.1158/1078-0432.CCR-10-3137. Epub 2011 Jun 1.PMID: 21632860
- Deans ZC, Costa JL, Cree I, Dequeker E, Edsjö A, Henderson S, Hummel M, Ligtenberg MJ, Loddo M, Machado JC, Marchetti A, Marquis K, Mason J, Normanno N, Rouleau E, Schuuring E, Snelson KM, Thunnissen E, Tops B, Williams G, van Krieken H, Hall JA; IQN Path ASBL. Integration of next-generation sequencing in clinical diagnostic molecular pathology laboratories for analysis of solid tumours; an expert opinion on behalf of IQN Path ASBL. Virchows Arch. 2017 Jan;470(1):5-20. doi: 10.1007/s00428-016-2025-7. Epub 2016 Sep 27. PMID: 27678269; PMCID: PMC5243883.
- Marchetti A, Barberis M, Papotti M, Rossi G, Franco R, Malatesta S, Buttitta F, Ardizzoni A, Crinò L, Gridelli C, Taddei GL, Clemente C, Scagliotti G, Normanno N, Pinto C. ALK rearrangement testing by FISH analysis in non-small- cell lung cancer patients: results of the first italian external quality assurance scheme. J Thorac Oncol. 2014 Oct;9(10):1470-6. doi: 10.1097/JTO.0000000000000280. PMID: 25170637.
- Marchetti A, Del Grammastro M, Felicioni L, Malatesta S, Filice G, Centi I, De Pas T, Santoro A, Chella A, Brandes AA, Venturino P, Cuccurullo F, Crinò L, Buttitta F. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: toward a real-time liquid biopsy for treatment. PLoS One. 2014 Aug 19;9(8):e103883. doi: 10.1371/journal.pone.0103883. PMID: 25137181; PMCID: PMC4138040.
- Buttitta F, Felicioni L, Del Grammastro M, Filice G, Di Lorito A, Malatesta S, Viola P, Centi I, D'Antuono T, Zappacosta R, Rosini S, Cuccurullo F, Marchetti A. Effective assessment of egfr mutation status in bronchoalveolar lavage and pleural fluids by next-generation sequencing. Clin Cancer Res. 2013 Feb 1;19(3):691-8. doi: 10.1158/1078-0432.CCR-12-1958. Epub 2012 Dec 14. PMID: 23243218
- Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, Camplese PP, Iarussi T, Mucilli F, Mezzetti A, Cuccurullo F, Sacco R, Buttitta F. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005 Feb 1;23(4):857-65. doi: 10.1200/JCO.2005.08.043. PMID: 15681531.
- Buttitta F, Pellegrini C, Marchetti A, Gadducci A, Cosio S, Felicioni L, Barassi F, Salvatore S, Martella C, Coggi G, Bosari S. Human telomerase reverse transcriptase mRNA expression assessed by real-time reverse transcription polymerase chain reaction predicts chemosensitivity in patients with ovarian carcinoma. J Clin Oncol. 2003 Apr 1;21(7):1320-5. doi: 10.1200/JCO.2003.09.065. PMID: 12663721.
- Kerr KM, Dafni U, Schulze K, Thunnissen E, Bubendorf L, Hager H, Finn S, Biernat W, Vliegen L, Losa JH, Marchetti A, Cheney R, Warth A, Speel EJ, Blackhall F, Monkhorst K, Jantus Lewintre E, Tischler V, Clark C, Bertran-Alamillo J, Meldgaard P, Gately K, Wrona A, Vandenberghe P, Felip E, De Luca G, Savic S, Muley T, Smit EF, Dingemans AC, Priest L, Baas P, Camps C, Weder W, Polydoropoulou V, Geiger TR, Kammler R, Sumiyoshi T, Molina MA, Shames DS, Stahel RA, Peters S; ETOP Lungscape Consortium. Prevalence and clinical association of gene mutations through multiplex mutation testing in patients with NSCLC: results from the ETOP Lungscape Project. Ann Oncol. 2018 Jan 1;29(1):200-208. doi: 10.1093/annonc/mdx629.PMID: 29186353
- Capoluongo E, La Verde N, Barberis M, Bella MA, Buttitta F, Carrera P, Colombo N, Cortesi L, Gion M, Guarneri V, Lorusso D, Marchetti A, Marchetti P, Normanno N, Pasini B, Pensabene M, Pignata S, Radice P, Ricevuto E, Sapino A, Tagliaferri P, Tassone P, Trevisiol C, Truini M, Varesco L, Russo A, Gori S. BRCA1/2 Molecular Assay for Ovarian Cancer Patients: A Survey through Italian Departments of Oncology and Molecular and Genomic Diagnostic Laboratories. Diagnostics (Basel). 2019 Oct 9;9(4):146. doi: 10.3390/diagnostics9040146.PMID: 31600986
Molecular diagnostics in oncology
This branch is mainly devoted to the development of diagnostic algorithms for the selection of oncological patients to be treated with targeted therapies.
Starting with the publication of the first seminal paper on the prevalence of EGFR mutations in Non Small Cell Lung Cancer (NSCLC) that allowed the selection of patients and tumor categories for anti-EGFR targeted treatments, the group has continued to operate in this area reporting important achievements for the development of diagnostic algorithms that are now internationally recognized. In particular, diagnostics algorithms for the selection of NSCLC patients carrying EGFR gene mutations, ALK1 and ROS1 gene fusions, have been produced. New generation therapies for these targets are increasingly effective and with fewer side effects, so no patient should be left behind without an appropriated treatment. A correct target treatment implies the identification of specific biomarkers alterations and specific patients categories. The algorithms we have developed can allow an accurate selection of patients not leaving behind potential responders. All these algorithms have been tested on large clinical series.
The detection of specific genomic alterations in particular subtypes of patients has allowed further insight in the pathological and molecular knowledge of these specific neoplastic forms.
A new pathological algorithm for the detection of rare mutations affecting different cancer types has recently been proposed for treatment of patients with tumor agnostic therapeutic approaches. This latter algorithm will introduce an innovative pathological workflow to be diffuse in pathology departments worldwide.
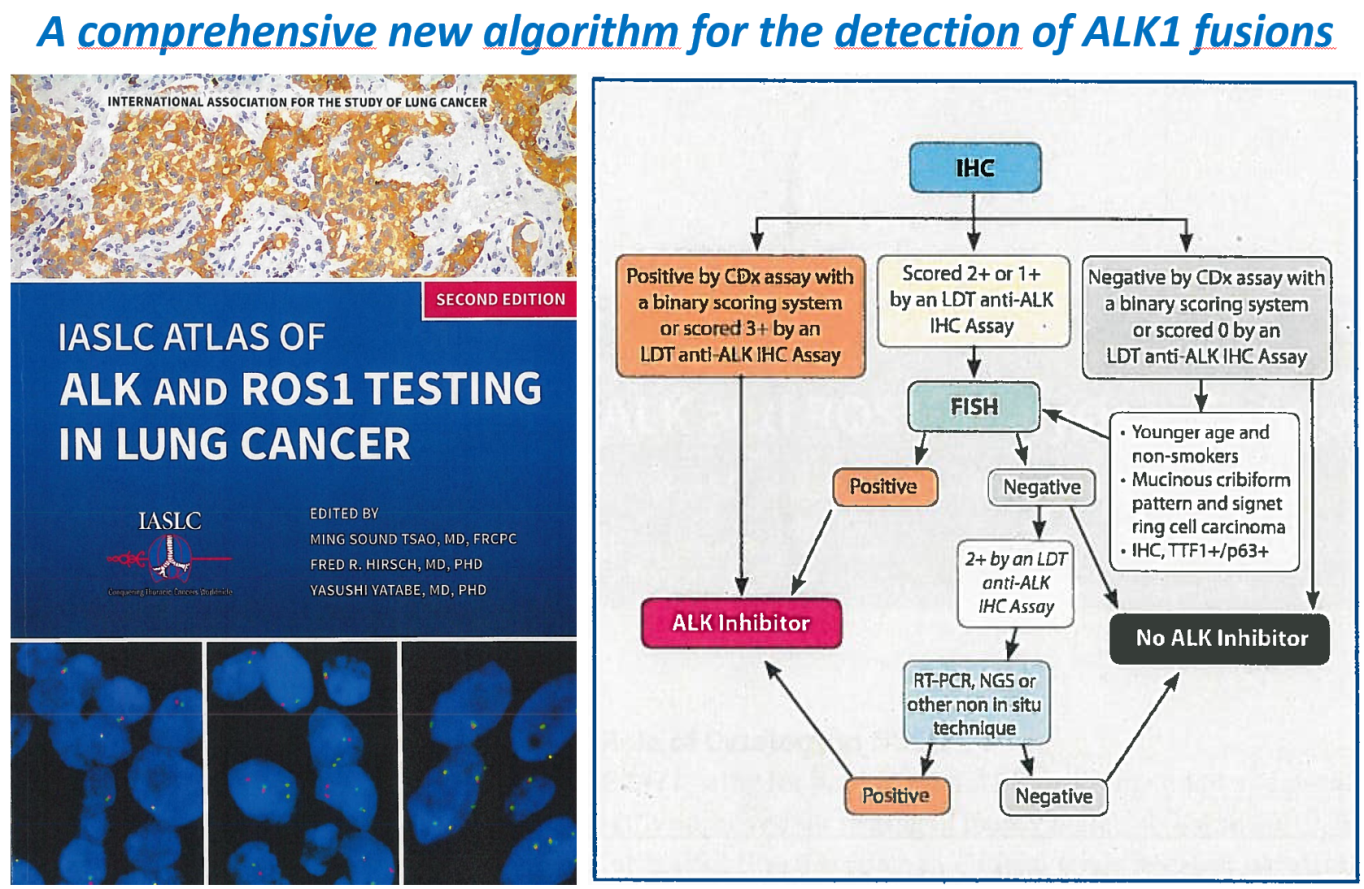
Comprehensive algorithm for the detection of Ros1 gene fusions
NTRK AND OTHER GENE MUTATIONS: A DIAGNOSTIC ALGORITHM
READ MORE
- Marchetti A, Felicioni L, Buttitta F. Assessing EGFR Mutations. N Engl J Med 2006 Feb 2;354(5):526-8. PMID: 16452569 DOI: 10.1056/NEJMc052564
- Buttitta F, Felicioni L, Lorito AD, Cortellini A, Irtelli L, Brocco D, Marino PD, Traisci D, D'Ostilio N, Paolo AD, Malorgio F, Assalone P, Felice SD, Fabbri F, Cianci G, Tursi M, Marchetti A. Early prediction of resistance to tyrosine kinase inhibitors by plasma monitoring of EGFR mutations in NSCLC: a new algorithm for patient selection and personalized treatment. Oncotarget. 2020 Mar 17;11(11):982-991. doi: 10.18632/oncotarget.27517. PMID: 32215186; PMCID: PMC7082112.
- Bubendorf L, Büttner R, Al-Dayel F, Dietel M, Elmberger G, Kerr K, López-Ríos F, Marchetti A, Öz B, Pauwels P, Penault-Llorca F, Rossi G, Ryška A, Thunnissen E. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch. 2016 Nov;469(5):489-503. doi: 10.1007/s00428-016-2000-3. Epub 2016 Aug 17. PMID: 27535289; PMCID: PMC5082594
- Marchetti A, Di Lorito A, Pace MV, Iezzi M, Felicioni L, D'Antuono T, Filice G, Guetti L, Mucilli F, Buttitta F. ALK Protein Analysis by IHC Staining after Recent Regulatory Changes: A Comparison of Two Widely Used Approaches, Revision of the Literature, and a New Testing Algorithm. J Thorac Oncol. 2016 Apr;11(4):487-95. doi: 10.1016/j.jtho.2015.12.111. Epub 2016 Feb 22. PMID: 26916631.
- Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, Pham L, Wang MM, Karachaliou N, Cao MG, Manzano JL, Ramirez JL, Torres JM, Buttitta F, Rudin CM, Collisson EA, Algazi A, Robinson E, Osman I, Muñoz-Couselo E, Cortes J, Frederick DT, Cooper ZA, McMahon M, Marchetti A, Rosell R, Flaherty KT, Wargo JA, Bivona TG. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015 Mar;47(3):250-6. doi: 10.1038/ng.3218. Epub 2015 Feb 9. PMID: 25665005; PMCID: PMC4930244.
- Blackhall FH, Peters S, Bubendorf L, Dafni U, Kerr KM, Hager H, Soltermann A, O'Byrne KJ, Dooms C, Sejda A, Hernández-Losa J, Marchetti A, Savic S, Tan Q, Thunnissen E, Speel EJ, Cheney R, Nonaka D, de Jong J, Martorell M, Letovanec I, Rosell R, Stahel RA. Prevalence and clinical outcomes for patients with ALK-positive resected stage I to III adenocarcinoma: results from the European Thoracic Oncology Platform Lungscape Project. J Clin Oncol. 2014 Sep 1;32(25):2780-7. doi: 10.1200/JCO.2013.54.5921. Epub 2014 Jul 28. PMID:25071109.
- Cazzoli R, Buttitta F, Di Nicola M, Malatesta S, Marchetti A, Rom WN, Pass HI. microRNAs derived from circulating exosomes as noninvasive biomarkers for screening and diagnosing lung cancer. J Thorac Oncol. 2013 Sep;8(9):1156-62. doi: 10.1097/JTO.0b013e318299ac32. PMID: 23945385; PMCID: PMC4123222.
- Marchetti A, Ardizzoni A, Papotti M, Crinò L, Rossi G, Gridelli C, Barberis M, Maiorano E, Normanno N, Taddei GL, Scagliotti G, Clemente C, Pinto C. Recommendations for the analysis of ALK gene rearrangements in non-small-cell lung cancer: a consensus of the Italian Association of Medical Oncology and the Italian Society of Pathology and Cytopathology. J Thorac Oncol. 2013 Mar;8(3):352-8. doi: 10.1097/JTO.0b013e31827d5280. PMID: 23407559.
- Marchetti A, Felicioni L, Malatesta S, Grazia Sciarrotta M, Guetti L, Chella A, Viola P, Pullara C, Mucilli F, Buttitta F. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol. 2011 Sep 10;29(26):3574-9. doi: 10.1200/JCO.2011.35.9638. Epub 2011 Aug 8. PMID:21825258.
- Marchetti A, Felicioni L, Pelosi G, Del Grammastro M, Fumagalli C, Sciarrotta M, Malatesta S, Chella A, Barassi F, Mucilli F, Camplese P, D'Antuono T, Sacco R, Buttitta F. Frequent mutations in the neurotrophic tyrosine receptor kinase gene family in large cell neuroendocrine carcinoma of the lung. Hum Mutat. 2008 May;29(5):609-16. doi: 10.1002/humu.20707. PMID: 18293376.
- Barbareschi M, Buttitta F, Felicioni L, Cotrupi S, Barassi F, Del Grammastro M, Ferro A, Dalla Palma P, Galligioni E, Marchetti A. Different prognostic roles of mutations in the helical and kinase domains of the PIK3CA gene in breast carcinomas. Clin Cancer Res. 2007 Oct 15;13(20):6064-9. doi: 10.1158/1078-0432.CCR-07-0266. PMID: 17947469.
- Buttitta F, Barassi F, Fresu G, Felicioni L, Chella A, Paolizzi D, Lattanzio G, Salvatore S, Camplese PP, Rosini S, Iarussi T, Mucilli F, Sacco R, Mezzetti A, Marchetti A. Mutational analysis of the HER2 gene in lung tumors from Caucasian patients: mutations are mainly present in adenocarcinomas with bronchioloalveolar features. Int J Cancer. 2006 Dec 1;119(11):2586-91. doi: 10.1002/ijc.22143. PMID: 16988931.
- Buttitta F, Felicioni L, Barassi F, Martella C, Paolizzi D, Fresu G, Salvatore S, Cuccurullo F, Mezzetti A, Campani D, Marchetti A. PIK3CA mutation and histological type in breast carcinoma: high frequency of mutations in lobular carcinoma. J Pathol. 2006 Feb;208(3):350-5. doi: 10.1002/path.1908. PMID: 16353168.
- Buttitta F, Martella C, Barassi F, Felicioni L, Salvatore S, Rosini S, D'Antuono T, Chella A, Mucilli F, Sacco R, Mezzetti A, Cuccurullo F, Callahan R, Marchetti A. Int6 expression can predict survival in early-stage non-small cell lung cancer patients. Clin Cancer Res. 2005 May 1;11(9):3198-204. doi: 10.1158/1078-0432.CCR-04-2308. PMID: 15867213.
- Marchetti A, Barassi F, Martella C, Chella A, Salvatore S, Castrataro A, Mucilli F, Sacco R, Buttitta F. Down regulation of high in normal-1 (HIN-1) is a frequent event in stage I non-small cell lung cancer and correlates with poor clinical outcome. Clin Cancer Res. 2004 Feb 15;10(4):1338-43. doi: 10.1158/1078-0432.ccr-1174-03. PMID: 14977834.
- Marchetti A, Tinari N, Buttitta F, Chella A, Angeletti CA, Sacco R, Mucilli F, Ullrich A, Iacobelli S. Expression of 90K (Mac-2 BP) correlates with distant metastasis and predicts survival in stage I non-small cell lung cancer patients. Cancer Res. 2002 May 1;62(9):2535-9. PMID: 11980646.
- Marchetti A, Pellegrini C, Buttitta F, Falleni M, Romagnoli S, Felicioni L, Barassi F, Salvatore S, Chella A, Angeletti CA, Roncalli M, Coggi G, Bosari S. Prediction of survival in stage I lung carcinoma patients by telomerase function evaluation. Lab Invest. 2002 Jun;82(6):729-36. doi: 10.1097/01.lab.0000017165.26718.60. PMID: 12065683.
- Marchetti A, Buttitta F, Bertacca G, Zavaglia K, Bevilacqua G, Angelucci D, Viacava P, Naccarato A, Bonadio A, Barassi F, Felicioni L, Salvatore S, Mucilli F. mRNA markers of breast cancer nodal metastases: comparison between mammaglobin and carcinoembryonic antigen in 248 patients. J Pathol. 2001Sep;195(2):186-90. doi: 10.1002/path.943. PMID: 11592097.
- Marchetti A, Bertacca G, Buttitta F, Chella A, Quattrocolo G, Angeletti CA, Bevilacqua G. Telomerase activity as a prognostic indicator in stage I non-small cell lung cancer. Clin Cancer Res. 1999 Aug;5(8):2077-81. PMID: 10473089.
- Gori S, Barberis M, Bella MA, Buttitta F, Capoluongo E, Carrera P, Colombo N, Cortesi L, Genuardi M, Gion M, Guarneri V, Incorvaia L, La Verde N, Lorusso D, Marchetti A, Marchetti P, Normanno N, Pasini B, Pensabene M, Pignata S, Radice P, Ricevuto E, Sapino A, Tagliaferri P, Tassone P, Trevisiol C, Truini M, Varesco L, Russo A; AIOM-SIGU-SIBIOC-SIAPEC-IAP Working Group. Recommendations for the implementation of BRCA testing in ovarian cancer patients and their relatives. Crit Rev Oncol Hematol. 2019 Aug;140:67-72. doi: 10.1016/j.critrevonc.2019.05.012. Epub 2019 May 25.PMID: 31176273.
- Bubendorf L, Dafni U, Schöbel M, Finn SP, Tischler V, Sejda A, Marchetti A, Thunnissen E, Verbeken EK, Warth A, Sansano I, Cheney R, Speel EJM, Nonaka D, Monkhorst K, Hager H, Martorell M, Savic S, Kerr KM, Tan Q, Tsourti Z, Geiger TR, Kammler R, Schulze K, Das-Gupta A, Shames D, Peters S, Stahel RA; Lungscape Consortium. Prevalence and clinical association of MET gene overexpression and amplification in patients with NSCLC: Results from the European Thoracic Oncology Platform (ETOP) Lungscape project. Lung Cancer. 2017 Sep;111:143-149. doi: 10.1016/j.lungcan.2017.07.021. Epub 2017 Jul 22.PMID: 28838386.
- Pirker R, Herth FJ, Kerr KM, Filipits M, Taron M, Gandara D, Hirsch FR, Grunenwald D, Popper H, Smit E, Dietel M, Marchetti A, Manegold C, Schirmacher P, Thomas M, Rosell R, Cappuzzo F, Stahel R; European EGFR Workshop Group. Consensus for EGFR mutation testing in non-small cell lung cancer: results from a European workshop. J Thorac Oncol. 2010 Oct;5(10):1706-13. doi: 10.1097/JTO.0b013e3181f1c8de.
- Kerr KM, Bubendorf L, Edelman MJ, Marchetti A, Mok T, Novello S, O'Byrne K, Stahel R, Peters S, Felip E; Panel Members; Panel Members. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol. 2014 Sep;25(9):1681-90. doi: 10.1093/annonc/mdu145. Epub 2014 Apr 8.
Digital-Quantitative pathology for immunotherapy.
Recent developments in digital imaging processing have revolutionized the pathological approach to the assessment of a series of important tumor biomarkers, including PD-L1 a key molecule in the selection of patients for treatment with Immune Checkpoint inhibitors (ICI). ICI are monoclonal antibodies recently entered in the clinical practice for treatment of patients with melanoma, non-small cell lung carcinoma (NSCLC) and renal cell carcinoma, while they are under investigation for many other indications. ICI belong to the category of immunotherapies, as they act on tumor microenvironment disinhibiting the host immune system in order to increase the immune response to tumor cells. Anti-PD-1 such as Nivolumab and Pembrolizumab are currently the most used ICI in clinical practice.
These drugs act against the programmed death-1 receptor (PD1) blocking its interaction with the
PD-L1 and PD-L2 ligands. These ligands are physiologically expressed on the surface of different cell types, including tumor cells and immune cells present in the tumor microenvironment. The PD-1 receptor is a negative regulator of T-cell activity leading to inhibition of T-cell proliferation and cytokine secretion. The advent of anti-PD1 drugs has led to a revolution in therapeutic algorithms for NSCLC patients. However, both the economic policies of health systems and the class of oncologists involved in daily clinical practice are negatively affected by the lack of selection criteria, ie the lack of biomarkers to select patients who can benefit of these new treatments. Immunohistochemical (IHC) evaluation of PD-L1 in tumor cells and/or in the inflammatory infiltrate, is at the moment the only validated parameter. IHC evaluation of PD-L1 has found routine clinical application in the treatment of NSCLC patients. In this setting, the expression of PD-L1 is particularly important, especially for first line treatment of patients with a high expression threshold (≥50%). However, since in the last years different anti-PD-L1 diagnostic clones have been used in different clinical trials, a number of different commercial kits are now available for the evaluation of PD-L1 expression. All this has affected the reproducibility of the test in daily clinical practice, so that a number of studies have been conducted to harmonize the main tests adopted in the most important clinical trials, including an Italian AIOM-SIAPEC dedicated multicenter study, coordinated by Prof. Marchetti.
The interpretation of the PD-L1 test, due to the aforementioned complexity of analysis, is inevitably operator dependent, as the sample can be interpreted differently by different pathologists. The main difficulty consists in the fact that the sample can be accurately evaluated under the microscope only at high magnification on a limited reading field (the greater the magnification, the more the reading field is reduced), but the pathologist must supply a report relative to the whole analyzed area (corresponding to hundreds of high magnifications fields). All this inevitably leads to a degree of subjectivity, well known to pathologists.
The current possibility of scanning images at high definition allows to obtaining "digital slides”. PD-L1-positive cells can be easily recognized on digital slides at high magnification. The implementation of algorithms developed on digital images to accurately define the percentage of immune-reactive cells can allow a more accurate assessment of the PD-L1 status. In a recent ongoing multicenter trial (“Digital PD-L1), involving 10 referral Oncology Centers, we decided to apply these algorithms on digitized images, for a more accurate definition of the PD-L1 tumor proportion score (TPS) in NSCLC patients already subjected to immunotherapeutic treatment with anti-PD-1 drugs with long follow-up data (Marchetti et al, manuscript in preparation). A new artificial intelligence- based algorithm has successfully been used in this series of patients in order to correlate PD-L1 expression with clinical follow-up data.

READ MORE
- Marchetti A, Di Lorito A, Buttitta F. Why anti-PD1/PDL1 therapy is so effective? Another piece in the puzzle. J Thorac Dis. 2017 Dec;9(12):4863-4866. doi: 10.21037/jtd.2017.11.105. PMID: 29312678; PMCID: PMC5756957.
- Marchetti A, Barberis M, Franco R, De Luca G, Pace MV, Staibano S, Volante M, Buttitta F, Guerini-Rocco E, Righi L, D'antuono T, Scagliotti GV, Pinto C, De Rosa G, Papotti M. Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) Assays to Test PD-L1 Expression for NSCLC Patients to Be Treated with Immune Checkpoint Inhibitors. J Thorac Oncol. 2017 Nov;12(11):1654-1663. doi: 10.1016/j.jtho.2017.07.031. Epub 2017 Aug 14. PMID: 28818609.
- Kerr KM, Thunnissen E, Dafni U, Finn SP, Bubendorf L, Soltermann A, Verbeken E, Biernat W, Warth A, Marchetti A, Speel EM, Pokharel S, Quinn AM, Monkhorst K, Navarro A, Madsen LB, Radonic T, Wilson J, De Luca G, Gray SG, Cheney R, Savic S, Martorell M, Muley T, Baas P, Meldgaard P, Blackhall F, Dingemans AM, Dziadziuszko R, Vansteenkiste J, Weder W, Polydoropoulou V, Geiger T, Kammler R, Peters S, Stahel R; Lungscape Consortium. A retrospective cohort study of PD-L1 prevalence, molecular associations and clinical outcomes in patients with NSCLC: Results from the European Thoracic Oncology Platform (ETOP) Lungscape Project. Lung Cancer. 2019 May;131:95-103. doi: 10.1016/j.lungcan.2019.03.012. Epub 2019 Mar 15
- Thunnissen E, Kerr KM, Dafni U, Bubendorf L, Finn SP, Soltermann A, Biernat W, Cheney R, Verbeken E, Warth A, Marchetti A, Speel EM, Pokharel S, Quinn AM, Monkhorst K, Navarro A, Madsen LB, Tsourti Z, Geiger T, Kammler R, Peters S, Stahel RA; European Thoracic Oncology Platform Lungscape Consortium. Programmed death-ligand 1 expression influenced by tissue sample size. Scoring based on tissue microarrays' and cross-validation with resections, in patients with, stage I-III, non-small cell lung carcinoma of the European Thoracic Oncology Platform Lungscape cohort. Mod Pathol. 2020 May;33(5):792-801. doi: 10.1038/s41379-019-0383-9. Epub 2019 Nov 18.PMID: 31740722
Esperimental pathology
The experimental pathology branch is coordinated by Prof Manuela Iezzi who worked from the very beginning of her research career on the comparative pathology of murine tumor models and the immunological prevention and therapy of tumors for the characterization of murine cancer models and establishing fructuous, long lasting collaborations with the main Italian groups working in this field. In the last ten years we have further extended our research fields and collaborations to other aspects of oncological research.
Detailed analysis of the molecular origins of cancer provided, in the last century, new understanding of the nature of cell transformation and of the determinants of tumor development and progression. We have shown that multiple and sequential genetic changes occur in the course of cancer development and revealed the biochemical basis for the action of these various genes as well as their effects on cells behavior.
Defining these genetic changes and their role in tumorigenesis, progression and metastatic spread, also provides important markers for use in diagnosis and allows to assign tumors to clinically relevant prognostic or therapeutically responsive groups. Eventually, they will also provide important targets for novel therapies.
On the basis of this premise, we can divide our experimental pathology activity into the following lines action:
- Tumor immunology and anti-cancer immunotherapies.
In collaboration with the Research groups directed by Federica Cavallo in Turin, PierLuigi Lollini in Boulogne, Vincenzo Bronte in Verona we carried out researches in the following areas: base research in Tumor Immunology, pre-clinical definition and validation of anti-tumour cellular and DNA vaccines, adoptive T-cell therapy approaches, utilization of new adjuvants and immunomodulators of immune reaction.
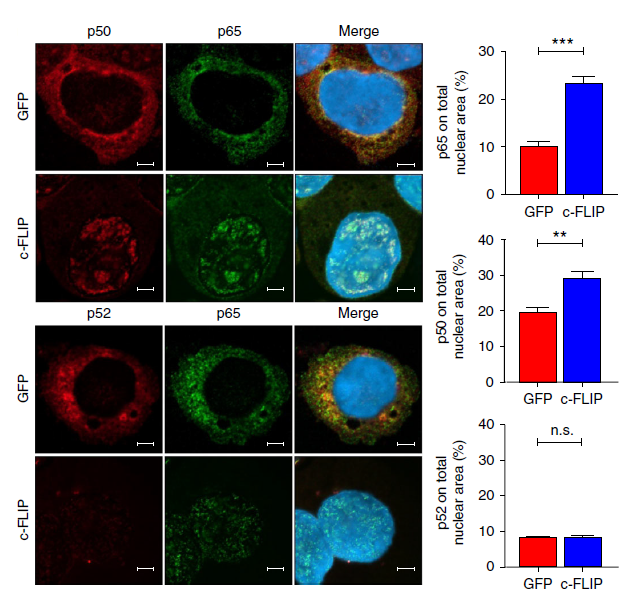
In Figure: Immunofluorescence confocal microscopy of p65 (red), p50 and p52 (green) nuclear translocation in transfected THP1 cells. From: Fiore A et al. Induction of immunosuppressive functions and NF-κB by FLIP in monocytes. Nat Commun. 2018 Dec 5;9(1):5193.
READ MORE
- Fiore A et al. Induction of immunosuppressive functions and NF-κB by FLIP in monocytes. Nat Commun. 2018 Dec 5;9(1):5193.
- Bartolacci C et al. Phage-Based Anti-HER2 Vaccination Can Circumvent Immune Tolerance against Breast Cancer. Cancer Immunol Res. 2018 Dec;6(12):1486-1498.
- Sara Sandri et al. Effective control of acute myeloid leukaemia and acute lymphoblastic leukaemia progression by telomerase specific adoptive T-cell therapy. Oncotarget 2017
- Bandini S et al. The non-inflammatory role of C1q during Her2/neu-driven mammary carcinogenesis. Oncoimmunology. 2016 Nov 8;5(12):e1253653.
- Sandri S et al. Feasibility of Telomerase-Specific Adoptive T-cell Therapy for B-cell Chronic Lymphocytic Leukemia and Solid Malignancies. Cancer Res. 2016 May 1;76(9):2540-51.
- Lamolinara A et al. Intradermal DNA Electroporation Induces Cellular and Humoral Immune Response and Confers Protection against HER2/neu Tumor. J Immunol Res. 2015; 2015:159145.
- Barutello G et al. Antitumor immunization of mothers delays tumor development in cancer-prone offspring. Oncoimmunology. 2015 Feb 3;4(5):e1005500. eCollection 2015 May.
- Croci S et al. Interleukin-15 is required for immunosurveillance and immunoprevention of HER2/neu-driven mammary carcinogenesis. Breast Cancer Res. 2015 May 22;17:70
- Macagno M et al. Multiple Roles of Perforin in Hampering ERBB-2 (Her-2/neu) Carcinogenesis in Transgenic Male Mice. J Immunol. 2014 Jun 1;192(11):5434-41.
- De Giovanni C et al. Vaccines against human HER2 prevent mammary carcinoma in mice transgenic for human HER2. Breast Cancer Res. 2014 Jan 23;16(1):R10.
- Silvio Bandini et al. Early onset and enhanced growth of autochthonous mammary carcinomas in C3 deficient Her2/neu transgenic mice. Oncoimmunology. 2013 Sep 1;2(9):e26137.
- Nanni P et al. Preclinical Therapy of Disseminated HER-2+ Ovarian and Breast Carcinomas with a HER-2-Retargeted Oncolytic Herpesvirus. PLoS Pathog. 2013 Jan;9(1):e1003155.
- De Giovanni C et al. Human responses against HER-2-positive cancer cells in human immune system-engrafted mice. British Journal of Cancer 2012 Oct 9;107(8):1302-9.
- Iezzi M et al. DNA vaccination against oncoantigens: A promise. Oncoimmunology. 2012 May 1;1(3):316-325.
- Arigoni M et al. A vaccine targeting angiomotin induces an antibody response which alters tumor vessel permeability and hampers the growth of established tumors. Angiogenesis. 2012 Jun;15(2):305-16.
- · Berta GN et al. A DNA vaccine against ERBB2 impairs chemical carcinogenesis in random-bred hamsters. Cancer Prev Res (Phila). 2011 Jul;4(7):994-1001.
- Rolla S et al. Erbb2 DNA vaccine combined with regulatory T cell deletion enhances antibody response and reveals latent low-avidity T cells: potential and limits of its therapeutic efficacy. J Immunol. 2010 Jun 1;184(11):6124-32.
- Quaglino E et al. A better immune reaction to Erbb-2 tumors is elicited in mice by DNA vaccines encoding rat/human chimeric proteins. Cancer Res. 2010 Apr 1;70(7):2604-12.
- Ugel S et al. Autoimmune B-cell lymphopenia after successful adoptive therapy with telomerase-specific T lymphocytes. Blood. 2010 Feb 18;115(7):1374-84.
- De Giovanni C et al. Tamoxifen combined to anti-HER-2/neu cell vaccine does not hamper cancer immunopreventive efficacy. Vaccine. 2009 Mar 23;27(14):2065-9.
- De Giovanni C et al. A multi-DNA preventive vaccine for p53/Neu-driven cancer syndrome. Hum Gene Ther. 2009 May;20(5):453-64.
- Molecular mechanisms of tumor development and progression.
In collaboration with national and international research groups we deepened the study of the in vivo effects on tumor onset, progression, aggressiveness and therapeutic response of several molecular alterations.
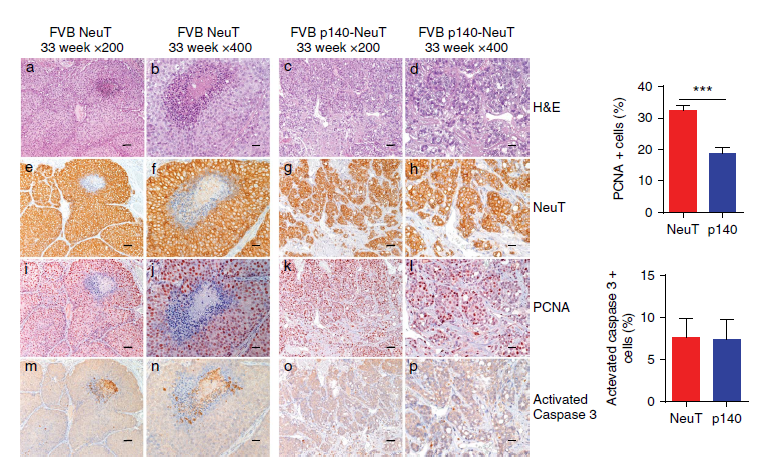
In Figure: Paraffin-embedded sections from FVB NeuT and FVB p140-NeuT tumors taken from mice at 33 weeks of age, analysed for hematoxylin–eosin (H&E), and for immunohistochemistry with antibodies to Her2Neu, PCNA and Activated Caspase 3. From: Grasso S et al. The scaffold protein p140Cap limits ERBB2-mediated breast cancer progression interfering with Rac GTPase-controlled circuitries. Nat Commun. 2017 Mar 16;8:14797.
READ MORE
- Grasso S et al. The SRCIN1/p140Cap adaptor protein negatively regulates the aggressiveness of neuroblastoma. Cell Death Differ. 2019 Jul 8. doi: 10.1038/s41418-019-0386-6.
- De Cola A et al. MiR-205-5p inhibition by locked nucleic acids impairs metastatic potential of breast cancer cells. Cell Death Dis. 2018 Jul 26;9(8):821.
- Castiello L et al. Disruption of IFN-I signaling promotes HER2/neu tumor progression and breast cancer stem cells. Cancer Immunol Res. 2018 Jun;6(6):658-670.
- Palladini A et al. HER2 isoforms co-expression differently tunes mammary tumor phenotypes affecting onset, vasculature and therapeutic response. Oncotarget. 2017 Apr 13.
- Grasso S et al. The scaffold protein p140Cap limits ERBB2-mediated breast cancer progression interfering with Rac GTPase-controlled circuitries. Nat Commun. 2017 Mar 16;8:14797.
- Andreani C et al. Resveratrol fuels HER2 and ERα-positive breast cancer behaving as proteasome inhibitor. Aging (Albany NY). 2017 Feb 26;9(2):508-523.
- Castagnoli L et al. Pathobiological implications of the d16HER2 splice variant for stemness and aggressiveness of HER2-positive breast cancer. Oncogene. 2017 Mar 23;36(12):1721-1732.
- Tilio M et al. Irreversible inhibition of Δ16HER2 is necessary to suppress Δ16HER2-positive breast carcinomas resistant to Lapatinib. Cancer Lett. 2016 Oct 10;381(1):76-84.
- Chikh A et al. Class II phosphoinositide 3-kinase C2β regulates a novel signaling pathway involved in breast cancer progression. Oncotarget. 2016 Feb 26.
- Manservisi F et al. Effect of maternal exposure to endocrine disrupting chemicals on reproduction and mammary gland development in female sprague-dawley rats. Reprod Toxicol. 2014 Dec 29.
- Steven A et al. HER-2/neu mediates oncogenic transformation via altered CREB expression and function. Mol Cancer Res. 2013 Sep 11.
- Bisaro B et al. p130Cas/Cyclooxygenase-2 axis in the control of mesenchymal plasticity of breast cancer cells. Breast Cancer Res. 2012 Oct 26;14(5):R137.
- Marchini C et al. The human splice variant Δ16HER2 induces rapid tumor onset in a reporter transgenic mouse. PLoS One. 2011 Apr 29;6(4):e18727.
- Cabodi S et al. p130Cas is an essential transducer element in ErbB2 transformation. FASEB J. 2010 Oct;24(10):3796-808.
- Ciraolo E et al. Essential role of the p110beta subunit of phosphoinositide 3-OH kinase in male fertility. Mol Biol Cell. 2010 Mar 1;21(5):704-11.
- Identification and validation of new prognostic and predictive markers.
Cancer clinically represents a heterogeneous disease. In order to obtain a more individualized and optimized therapy is necessary to integrate prognostic and predictive markers in treatment decisions. To identify patients at risk of relapse after surgery and who may benefit most from chemotherapy or to predict whether a tumor will respond to a particular chemotherapy, offers the possibility to minimize overtreatment, under-treatment, and incorrect treatment and to define which therapy offers the best efficacy.
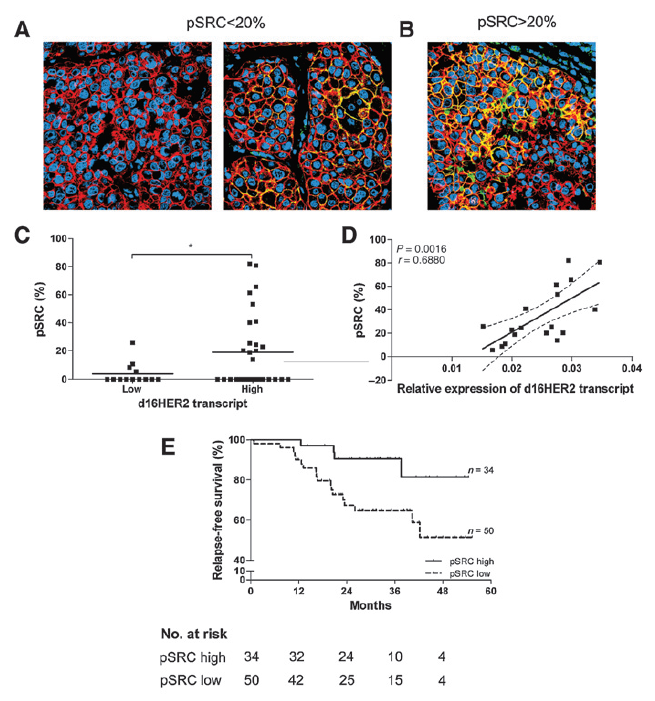
In Figure: Expression and coexpression of HER2 and pSRC markers and association between d16HER2 transcript and pSRC expression and risk of relapse in human HER2-overexpressing breast cancers patients treated with trastuzumab. From: Castagnoli L et al. Activated d16HER2 homodimers and SRC kinase mediate optimal efficacy for trastuzumab. Cancer Res. 2014 Nov 1;74(21):6248-59.
READ MORE
- Lattanzio R et al. PLC-gamma-1 phosphorylation status is prognostic of metastatic risk in patients with early-stage Luminal-A and -B breast cancer subtypes. BMC Cancer. 2019 Jul 30;19(1):747.
- Corda G et al. Functional and prognostic significance of the genomic amplification of frizzled 6 (FZD6) in breast cancer. J Pathol. 2017 Feb;241(3):350-361.
- Marchetti A et al. ALK Protein Analysis by IHC Staining after Recent Regulatory Changes: A Comparison of Two Widely Used Approaches, Revision of the Literature, and a New Testing Algorithm. J Thorac Oncol. 2016 Apr;11(4):487-95.
- Castagnoli L et al. Activated d16HER2 homodimers and SRC kinase mediate optimal efficacy for trastuzumab. Cancer Res. 2014 Nov 1;74(21):6248-59
- Chiari R et al. Dramatic response to crizotinib in ROS1 fluorescent in situ hybridization- and immunohistochemistry-positive lung adenocarcinoma: a case series. Clin Lung Cancer. 2014 Nov;15(6):470-4.
- Marchini C et al. Mesenchymal/stromal gene expression signature relates to basal-like breast cancers, identifies bone metastasis and predicts resistance to therapies. PLoS One. 2010 Nov 30;5(11):e14131.
- Identification, validation and preclinical testing of new targets for anticancer therapy, new drugs and methods for early diagnosis of cancer.
Several phases are necessary for new drug development before new drugs arrives to clinical trials. Our main activity in this field is preclinical testing: we analyze the bioactivity, safety, and efficacy of the new formulated drug product. We also collaborated with researchers in radiology to develop innovative tools for an early, more precise and noninvasive diagnosis. Strict interdisciplinary collaborations are essentials to develop and validate new radiological methods.
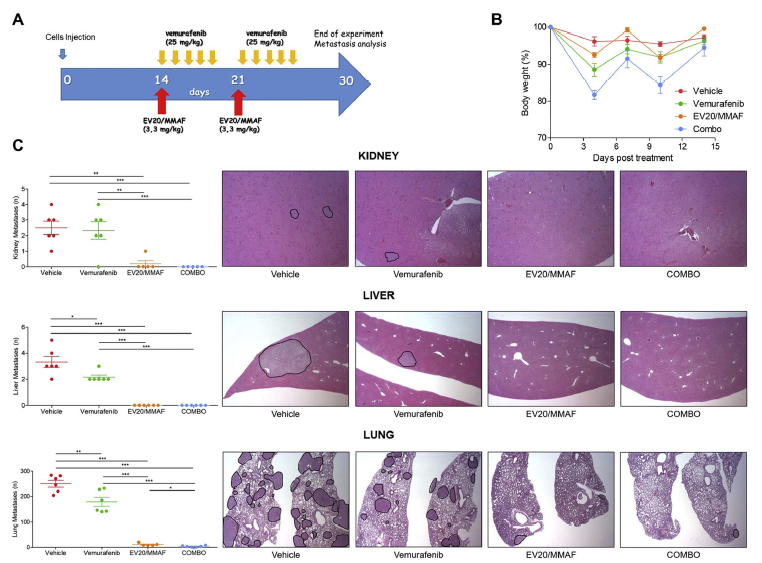
In Figure: EV20/MMAF shows superior activity of vemurafenib in vivo. From: Capone E et al. EV20-mediated delivery of cytotoxic auristatin MMAF exhibits potent therapeutic efficacy in cutaneous melanoma. J Control Release. 2018 Mar 14.
READ MORE
- Koschorke A et al. Phenethyl isothiocyanate hampers growth and progression of HER2-positive breast and ovarian carcinoma by targeting their stem cell compartment. Cell Oncol (Dordr). 2019 Aug 2
- Basile A et al. Development of an anti-BAG3 humanized antibody for treatment of pancreatic cancer. Mol Oncol. 2019 Jun;13(6):1388-1399.
- Giansanti F et al. Secreted Gal-3BP is a novel promising target for non-internalizing Antibody-Drug Conjugates. J Control Release. 2019 Jan 28;294:176-184.
- Ferrauto G et al. Generation of multiparametric MRI maps by using Gd-labelled- RBCs reveals phenotypes and stages of murine prostate cancer. Sci Rep. 2018 Jul 12;8(1):10567.
- Gambini V et al. In vitro and in vivo studies of gold(I) azolate/phosphane complexes for the treatment of basal like breast cancer. Eur J Med Chem. 2018 Jun 2;155:418-427.
- Capone E et al. EV20-mediated delivery of cytotoxic auristatin MMAF exhibits potent therapeutic efficacy in cutaneous melanoma. J Control Release. 2018 Mar 14.
- Capone E et al. EV20-Sap, a novel anti-HER-3 antibody-drug conjugate, displays promising antitumor activity in melanoma. Oncotarget. 2017 Sep 8;8(56):95412-95424.
- Montani M et al. The water soluble ruthenium(II) organometallic compound [Ru(p-cymene)(bis(3,5 dimethylpyrazol-1-yl)methane)Cl]Cl suppresses triple negative breast cancer growth by inhibiting tumor infiltration of regulatory T cells. Pharmacol Res. 2016 Mar 30;107:282-290.
- Mosley M et al. Claudin-4 SPECT Imaging Allows Detection of Aplastic Lesions in a Mouse Model of Breast Cancer. J Nucl Med. 2015 May;56(5):745-51.
- Cornelissen B et al. Imaging DNA damage allows detection of preneoplasia in the BALB-neuT model of breast cancer. J Nucl Med. 2014 Dec;55(12):2026-31.
- Riccardo F et al. Characterization of a genetic mouse model of lung cancer: a promise to identify Non-Small Cell Lung Cancer therapeutic targets and biomarkers. BMC Genomics. 2014;15 Suppl 3:S1.
- Kalogris C et al. Sanguinarine suppresses basal-like breast cancer growth through dihydrofolate reductase inhibition. Biochem Pharmacol. 2014 May 27.
- Conti L et al. Optical imaging detection of microscopic mammary cancer in ErbB-2 transgenic mice through the DA364 probe binding αv β3 integrins. Contrast Media Mol Imaging. 2013 Jul-Aug;8(4):350-60.
- Nanni P et al. Multiorgan Metastasis of Human HER-2(+) Breast Cancer in Rag2(-/-);Il2rg(-/-) Mice and Treatment with PI3K Inhibitor. PLoS One. 2012;7(6):e39626. Epub 2012 Jun 21.
- Iezzi M et al. HCG hastens both the development of mammary carcinoma and the metastatization of HCG/LH and ERBB-2 receptor-positive cells in mice. Int J Immunopathol Pharmacol. 2011 Jul-Sep;24(3):621-30.
- Pannellini T et al. A dietary tomato supplement prevents prostate cancer in TRAMP mice. Cancer Prev Res (Phila). 2010 Oct;3(10):1284-91.
- Bono AV et al. Sorafenib's inhibition of prostate cancer growth in transgenic adenocarcinoma mouse prostate mice and its differential effects on endothelial and pericyte growth during tumor angiogenesis. Anal Quant Cytol Histol. 2010 Jun;32(3):136-45.
- De Santis R et al. AvidinOX for highly efficient tissue-pretargeted radionuclide therapy. Cancer Biother Radiopharm. 2010 Apr;25(2):143-8.
- Coscia M et al. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. 2010 Dec;14(12):2803-15.
- Drug repositioning.
Drug repositioning, also known as drug repurposing, is a strategy to find new indications for existing drugs, often in diseases with very different profiles to that for which the drug was originally developed. Repurposing offers a number of significant advantages in comparison with de novo drug development: it can significantly reduce the cost and development time, extensive pharmacokinetic, pharmacodynamic and safety data are already available and as many compounds have demonstrated safety in humans, it often negates the need for phase I clinical trials.
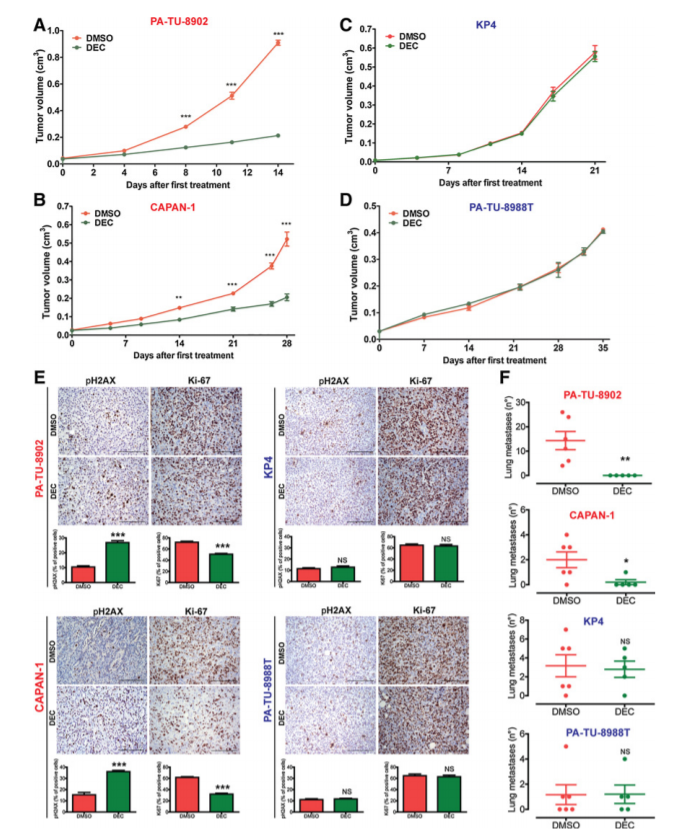
In Figure: Decitabine specifically inhibited growth and the development of lung metastases of KRAS-dep PDAC derived xenograft tumors. From: Mottini C et al. Predictive signatures inform the effective repurposing of Decitabine to treat K-RAS-dependent Pancreatic Ductal Adenocarcinoma. Cancer Res. 2019 Sep 5.
READ MORE
- Bibbo' S et al. Repurposing a psychoactive drug for children with cancer: p27Kip1-dependent inhibition of metastatic neuroblastomas by Prozac. Oncogenesis. 2020 Jan 2;9(1):3.
- Mottini C et al. Predictive signatures inform the effective repurposing of Decitabine to treat K-RAS-dependent Pancreatic Ductal Adenocarcinoma. Cancer Res. 2019 Sep 5.
- Carrella D et al. Computational drugs repositioning identifies inhibitors of oncogenic PI3K/AKT/P70S6K-dependent pathways among FDA-approved compounds. Oncotarget. 2016 Aug 16
- Inflammation
Our background expertise on functional morphology of cancer related immune and microenvironment modifications, naturally brought us to start collaborations with groups interested in diseases in which inflammation plays a cardinal role.

In Figure: Resolvin D1 is organ protective in chronic P. aeruginosa infection and enhances non phlogistic clearance of P. aeruginosa and inflammatory cells by macrophages. From: Codagnone M et al. Resolvin D1 enhances the resolution of lung inflammation caused by long-term Pseudomonas aeruginosa infection. Mucosal Immunol. 2017 Apr 19.
READ MORE
- Isopi E et al. Resolvin D1 Reduces Lung Infection and Inflammation Activating Resolution in Cystic Fibrosis. Front. Immunol., 27 April 2020.
- Matte A et al. Resolution of sickle cell disease-associated inflammation and tissue damage with 17R-resolvin D1. Blood. 2019 Jan 17;133(3):252-265.
- Giuliani C et al. Resveratrol has anti-thyroid effects both in vitro and in vivo. Food Chem Toxicol. 2017 Sep;107(Pt A):237-247.
- Codagnone M et al. Resolvin D1 enhances the resolution of lung inflammation caused by long-term Pseudomonas aeruginosa infection. Mucosal Immunol. 2017 Apr 19.
- Petrarca C et al. rBet v 1 immunotherapy of sensitized mice with Streptococcus thermophilus as vehicle and adjuvant. Hum Vaccin Immunother. 2014 Mar 6;10(5).
- Nubile M et al. Expression of CREB in Primary Pterygium and Correlation with Cyclin D1, ki-67, MMP7, p53, p63, Survivin and Vimentin. L. Ophthalmic Res. 2013;50(2):99-107.
- Nubile M et al. Pathological changes of the anatomical structure and markers of the limbal stem cell niche due to inflammation. Mol Vis. 2013;19:516-25. Epub 2013 Feb 26.
- Petrarca C et al. Monomeric allergoid intragastric administration induces local and systemic tolerogenic response involving IL-10-producing CD4(+)CD25(+) T regulatory cells in mice. Int J Immunopathol Pharmacol. 2010 Oct-Dec;23(4):1021-31.
IL-7 e kidney immunopathology
Professor Francesca Aiello ‘s research concerns studies sharing a long-standing interest in the immune system and cytokines. These studies include interleukin 7 (IL-7) signal transduction and hematopoietic functions, and kidney immunopathology.
Interleukin 7
(IL-7) is required for T cell differentiation and homeostasis, and genetic defects in IL-7 receptor components cause human severe combined immunodeficiency disease (SCID). On the other hand, mutations in IL-7 receptor play a leukemogenic role in acute T cell leukemia, and other lymphoid malignancies. In collaboration with Dr Scott Durum, Laboratory of Cancer Immunometabolism, National Cancer Institute, Frederick, Maryland, Usa, it has been demonstrated that IL-7 stimulation induced or increased the tyrosine phosphorylation of 16 proteins involved in pathways related to survival, proliferation and gene expression, including CrkL.
2-D western blot analysis: CrkL tyrosine phosphorylation in IL-7-stimulated murine D1 cells
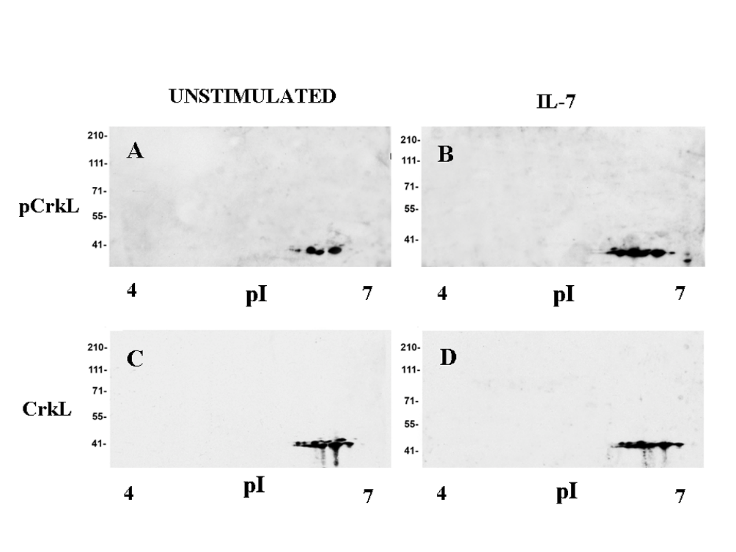
Crk-L is a member of the Crk family of adaptors, playing an important role in hematopoietic cells, and is highly phosphorylated in chronic myeloid leukemia. We are currently studying the role of Crk proteins in IL-7 signal transduction using different experimental models of conditionally deficient mice.
READ MORE
- Aiello FB, Guszczynski T, Li W, et al. IL-7-induced phosphorylation of the adaptor Crk-like and other targets. Cell Signal. 2018;47: 131‐141
- Centurione L, Aiello FB. DNA Repair and Cytokines: TGF-β, IL-6, and Thrombopoietin as Different Biomarkers of Radioresistance. Front Oncol. 2016; 6:175.
- Aiello FB, Graciotti L, Procopio AD, Keller JR, Durum SK. Stemness of T cells and the hematopoietic stem cells: fate, memory, niche, cytokines. Cytokine Growth Factor Rev. 2013;24: 485‐501.
- Zappacosta R, Aiello FB, D'Antuono T, et al. Detection of nuclear and membrane antigens by liquid-based cytology following long-term storage of d1 cells, karpas cells, and peripheral blood mononuclear cells. Ann Clin Lab Sci. 2011;41: 353‐359.
- Aiello FB, Keller JR, Klarmann KD, Dranoff G, Mazzucchelli R, Durum SK. IL-7 induces myelopoiesis and erythropoiesis. J Immunol. 2007; 178:1553‐1563.
Kidney Immunopathology:
In past years, several studies concerning human kidney transplant immunopathology have been performed in collaboration with Prof. Marialuisa Valente, Institute of Anatomic Pathology, Padua University, Italy. Glomerular diseases constitute some of the major problems in nephrology, and exhibit variable course ranging from benign conditions to progressive renal failure. At present, morphologic, morphomertric and immunohistochemical parameters in renal biopsies of patients affected by glomerular diseases are examined to identify useful prognostic markers.
READ MORE
- Viola P, Centurione L, Felaco P, Lattanzio G, D’Antuono T, Marcella Liberatore M, Di Pietro R, Ranelletti FO, Bonomini M and Aiello FB. Prognostic value of morphologic and morphometric analyses in IgA nephropathy biopsies. Translational Medicine Communications, 2016; 1:7:21
- Aiello FB, Furian L, Della Barbera M, et al. Glomerulitis and endothelial cell enlargement in C4d+ and C4d- acute rejections of renal transplant patients. Hum Pathol. 2012;43(12):2157‐2166.
- Aiello FB, Calabrese F, Rigotti P, et al. Acute rejection and graft survival in renal transplanted patients with viral diseases. Mod Pathol. 2004;17: 189‐196.
- Musiani P, Citterio F, Maggiano N, et al. Glucocorticoid inhibitory action on OKT3-stimulated peripheral blood lymphocytes from renal transplant patients. Transplantation. 1984;38: 309‐312.
Scientific training and positions of the group leader
- 2019-date, Group leader at the Cast Institute.
- 2019-Date, Chief, Laboratory of Molecular Diagnostics in Oncology
- 2010-2019: Director, Center of Predictive Molecular Medicine, University-Foundation, Chieti.
- 2014-date. Director of the Pathology Department, SS Annunziata Hospital/University of Chieti.
- 2008-date: Director, Adriatic Biobank, University of Chieti – ASL2 Chieti
- 2005-date: Full Professor of Pathology, University "G D'Annunzio", Chieti, Italy.
- 1998-2005: Associate Professor of Pathology, University "G D'Annunzio", Chieti, Italy.
- 1992-1998: Staff Researcher, University of Pisa. Italy.
- 1988-1990: Visiting Fellow, Laboratory of Tumor Immunology and Biology, NIH, Bethesda MD.
Awards
- Recipient of the PON Grant 2019-2021 ”DEVELOPMENT OF MECCATRONICH, GENOMC AND BIOINFORMATIC PATFORMS FOR PRECISION ONCOLOGY (PMBG)” Project Code ARS01_01195- Total Grant: Euro 3.945.273,99













