Flow Cytometry
INFO Service
The CAST Flow Cytometry service supports the activities related to flow cytometry analysis, cell sorting and imaging flow cytometry of the research groups within CAST and outsiders, also providing advanced expertise for experimental design, polychromatic antibody panel development, data acquisition and computational analysis. By closely collaborating with other groups within CAST and the University, the service develops flow cytometry technology to increase the analysis capability at the level of single cells. This service houses 3 analyzers allowing to make multiple measurements (up to 13 parameters at the same time, that can be either molecular or biological) on a high number of cells per seconds (> 10,000) at a single cell level, revealing the heterogeneity in cell populations hidden by other techniques. These carachteristics make flow cytometry the technology of choice to count cells and simultaneously analyze several parameters with high sensitivity in a very short period of time. Several flow cytometry applications are available: immunophenotyping, cell counting, detection of fluorescent proteins, cell cycle and DNA content analysis, telomere length measurement, Ca++ flux, cell viability, apoptosis and proliferation, cytokine detection analysis of rare events (i.e. circulating endothelial cells or circulating tumour cells), identification and subtyping of extracellular vesicles, virus and spermatozoa analyses. The service also provides expertise in flow cytometry applied to food safety (detection of antibiotics residues) and food quality control. The service has also access to a 10-parameter cell sorter that allows the identification and the physical separation of viruses, specific cell or extracellular vesicle subtypes for further analyses by other techniques (i.e. proteomics/lipidomics, genomics). Cell sorting also allows to separate, under sterile conditions, any cell subtype that can be finally put again in culture. The service also houses an ImageStream Imaging Flow Cytometer (IFC). It combines the speed, sensitivity, and phenotyping abilities of flow cytometry with the detailed imagery and morphological insights of fluorescence microscopy. The applications of imaging flow cytometry include analysis of apoptosis based on the changes in nuclear morphology, nuclear-cytoplasmic translocation, quantitative analysis of internalized bacteria and protozoan parasites and in recent years it was also employed to study the internalization of conjugated antibodies and PKH-labeled exosomes and microvesicles, as well as for the analysis of cell interactions, intercellular communication by exchange of cytoplasmic material, analysis of immune synapse. The flow cytometry service has access to three dedicated analysis workstations with: FCS Express V and FlowJo X and v.8.8.6 software for flow cytometry data analysis and IDEAS software for ImageStream data analysis.


INFO Access
The flow cytometry service provides to interested researchers any support to complete service projects in which cell preparation, staining, data acquisition and analysis are carried out entirely by our resource staff. The service also offers a free basic flow cytometry course for internal users who want to have access to flow cytometry analyzers and the cell sorter (mandatory).


FACS CANTO II Analyser (BD Biosciences)
The FACS Canto II (BD Biosciences) is a benchtop analyser for clinical and research applications. It is able to register up to 10,000 events/second. Equipped with three lasers: a 488 nm laser (Blue), a 633 nm laser (Red), and a 405 nm laser (violet), it allows the detection of the two classical scattered parameters (Side Scatter, SSC and Forward Scatter, FSC) and its optical system permits the symoultaneous acquisition of 8 fluorescent parameters. The details of the FASC Canto II optical system are listed in Table 1. Pulse processing (height, width and area) can be measured on all the parameters. It runs by using BD FACSDiva™ software, which allows advanced cell subset analysis, functional studies, as well as soluble (i.e. cytokines) and cell-surface protein quantification. Data can be saved as as FCS 2.0 or FCS 3.0 files. ESFRI classification: Health and Food Domain; Acquisition year: 2011.READ MORE
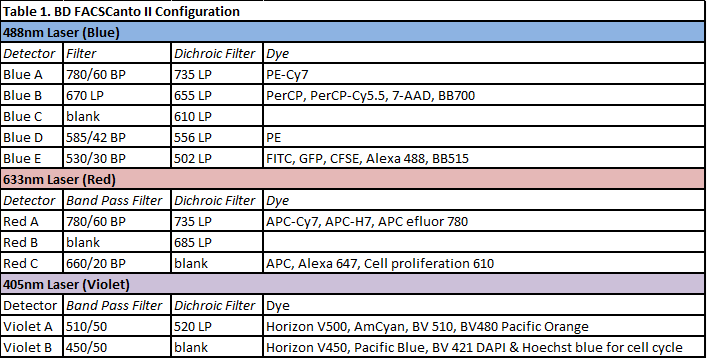
FACS Verse Analyser (BD Biosciences)
The FACSVerse (BD Biosciences) cytometer is an analyser for research use only. It registers up to 30,000 events/second and it is equipped with three lasers: a 488 nm laser (Blue), a 633 nm laser (Red), and a 405 nm laser (violet). It allows the detection of the two classical scattered parameters (Side Scatter, SSC and Forward Scatter, FSC) and its optical system permits the symoultaneous acquisition of 8 fluorescent parameters. Pulse processing (height, width and area) can be measured on all the parameters. It is equipped with miniaturized detection optics, and memory chips integrated in the filter/mirror units to automate configuration detection. This enables the system to alert users if the instrument configuration does not match the defined experimental parameters, ensuring the reliability of results. The details of the FACS Verse optical system are listed in Table 2. It runs by using BD FACSuite™ software, which allows advanced cell subset analysis, functional studies, as well as soluble and cell-surface protein quantification. Data can be saved as as FCS 2.0 or FCS 3.0 files. This analyser is also equipped with the BD™ Flow Sensor option for volumetric measurement that allows to register the volume over the entire acquisition time. It enables the user to reproducibly obtain a precise measure of cells or particles in a volume of sample acquired by the system. It directly determines the volume of the samples as it passes through the sample injection tubing for accurate counts. ESFRI classification: Health and Food Domain; Acquisition year: 2013.READ MORE
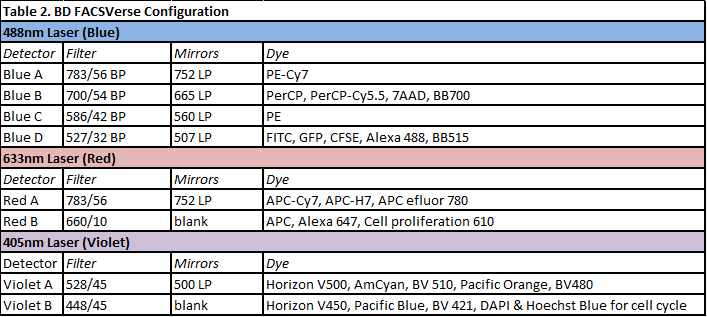
FACS Calibur Analyser (BD Biosciences)
The FACS Calibur is an analyser equipped with two lasers (a 488 nm Blue laser and a 633 nm Red laser), allowing to analyze FSC, SSC and 4 fluorescent paramters. Emission filters are fixed and can’t be changed (Table 3). Pulse processing (width and area) on one of fluorescence parameters can be done, but FL4 cannot be used in that case. Samples are acquired (10 bits digitalization – 4 decades) using the CellQuest software and are saved as FCS 2.0 files. ESFRI classification: Health and Food Domain; Acquisition year: 2003.READ MORE
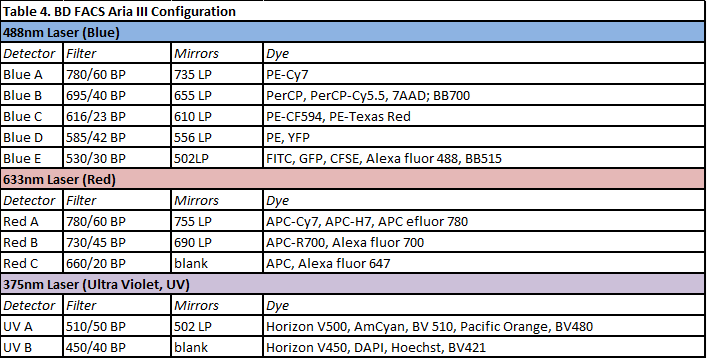
FACS Aria III Fluorescence Activated Cell Sorter (BD Biosciences)
The FACSAria III is equipped with 3 lasers, 10 fluorescence channels as well as Forward Scatter and Side Scatter channels. It registers up to 70.000 ev/sec. It is a state of the art cell sorter capable of physically separate 4 populations simultaneously. Sorted cells can be collected into tubes, plates, or onto slides, even in sterile conditions. Four different nozzles (70μm, 85μm, 100μm and 130μm) are available. It runs by using BD FACSDiva™ software. Data can be saved as as FCS 2.0 or FCS 3.0 files. ESFRI classification: Health and Food Domain; Acquisition year: 2011.READ MORE
Amnis ImageStreamX Mk II (Luminex Corporation)
The Amnis ImageStreamX Mk II (Luminex Corporation) is a multispectral imaging flow cytometer, able to combine advantages of flow cytometry (in terms of ability to detect cells in suspension, speed, sensitivity with a large number of sampling cells) with those of microscopy analysis (quantitative and qualitative features, localization of fluorescent probes, image analysis-based methods). The ImageStreamX Mk II can analyze up to 5000 cells/sec, ideal for rare cell analysis. This system is equipped with the MultiMag option that provides 20X and 60X objective lenses, in addition to the standard 40X objective, for greater experimental flexibility and improved resolution. It has a brightfield lamp, a 488nm and 642nm excitation lasers, being able to acquire up to 6 channels (Table 5). Precisely-controlled fluidics positions cells in the plane of focus and a velocity detection system synchronizes the CCD camera readout with the motion of the cells. It runs by using INSPIRE® Software. Data analysis is done with IDEAS® Software (in the ImageStream Workstation). IDEAS integrates image data, plots and statistics: the gallery shows images of every cell and the tabular data section allows to view population statistics as well as individual feature sets. Imaging in flow can be used to answer specific research questions such as apoptosis, cell cycle, co-localization, internalization, nuclear localization, shape change, spot counting. ESFRI classification: Health and Food Domain; Acquisition year: 2012.READ MORE
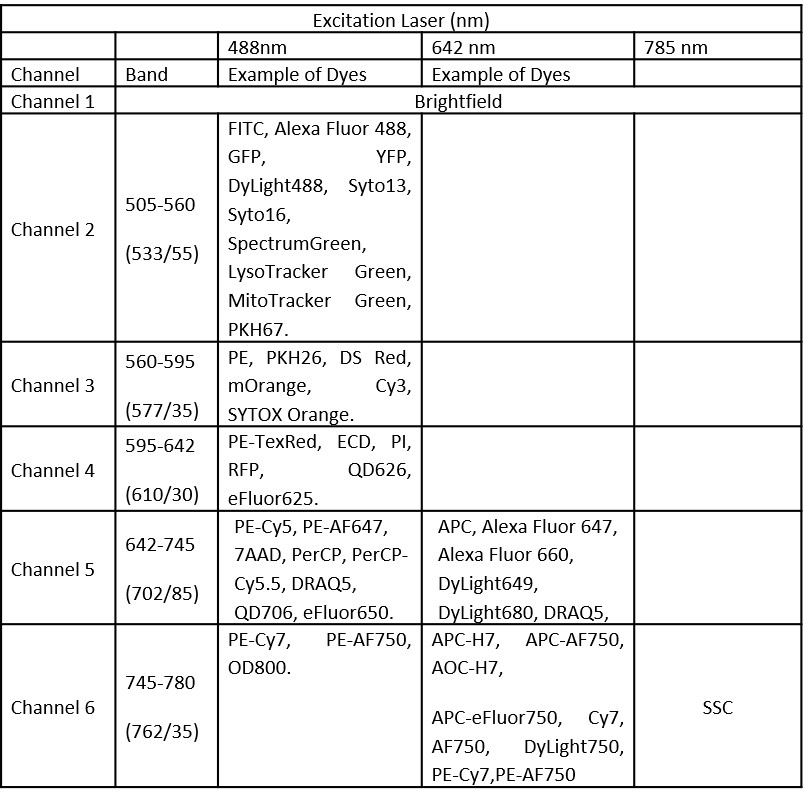
CytoFLEX (Beckman Coulter)
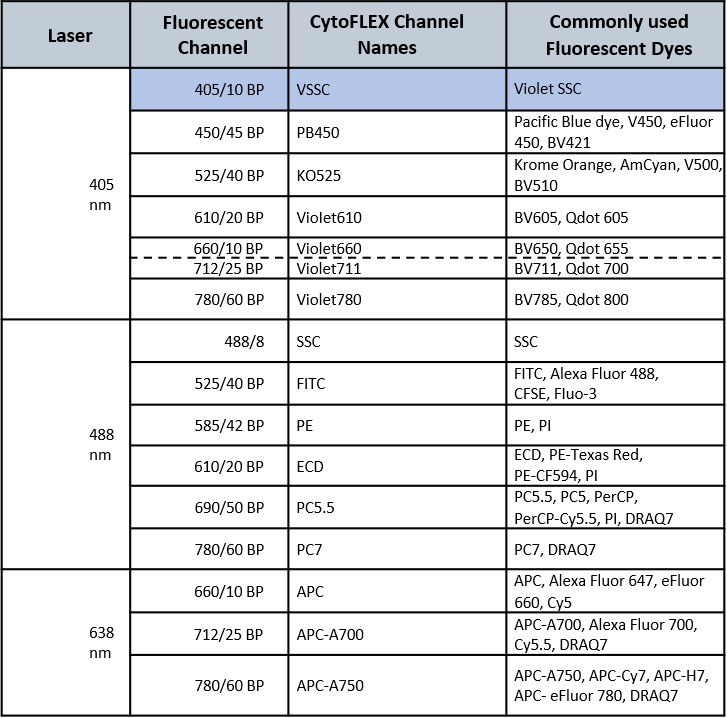
The CytoFLEX flow cytometer (Beckman Coulter) provides a sensitive platform with a high resolution on the side scatter channel thanks to the possibility of using the violet laser (405nm) for the detection of that parameter. The instrument is particularly suitable for the analysis of very small events (extracellular vesicles, apoptotic bodies, viruses, bacteria, yeasts, etc.). Using the Violet SSC parameter, the CytoFLEX is able to clearly discriminate 80 nm polystyrene and 150 nm silica nanoparticles from the background noise. The cytometer is equipped with 3 lasers, a violet (405 nm), a blue (488 nm) and a red (638 nm) laser with a 13-color configuration and the possibility to activate additional channels. For the detection of fluorescence the instrument uses the Avalanche Photodiode Detectors (APD), instead of the classic photomultipliers (PMT). A distinctive feature of the ADP is the high quantum efficiency of more than 80%, in particular for wavelengths greater than 800 nm. A module for plate reading (96 wells) is available on the instrument installed at the facility and is equipped with a volumetric counter for all acquisition speeds. The instrument is also equipped with CytoExpert 2.4 Software for acquisition and analysis. ESFRI classification: Health and Food Domain; Acquisition year: 2021.READ MORE
- Lanuti P., Bertagnolo V., Pierdomenico L., Bascelli A., Santavenere E., Alinari L., Capitani S., Miscia S., Marchisio M. Enhancement of TRAIL cytotoxicity by AG-490 in human ALL cells is characterized by downregulation of cIAP-1 and cIAP-2 through inhibition of Jak2/Stat3. Cell Res. 19:1079-1089, (2009). ISSN/ISBN: 1001/0602. IF: 8.151. 10.1038/cr.2009.80
- Lanuti P., Ciccocioppo F., Bonanni L., Marchisio M., Lachmann R., Tabet N., Pierdomenico L., Santavenere E., Catinella V., Iacone A., Thomas A., Gambi D., Miscia S., Onofrj M., Kern F. Amyloid-specific T-cells differentiate Alzheimer's disease from Lewy body dementia. Neurobiol Aging. 33(11):2599-611. (2012). ISSN: 0197-4580. IF:6.166. 10.1016/j.neurobiolaging.2012.01.004
- Lanuti P., Santilli F., Marchisio M., Pierdomenico L., Vitacolonna E., Santavenere E., Iacone A., Davì G., Romano M., Miscia S. A novel flow cytometric approach to distinguish circulating endothelial cells from endothelial microparticles: Relevance for the evaluation of endothelial dysfunction. J Immunol Methods. 29;380(1-2):16-22. (2012). ISSN: 0022-1759. IF: 2.225. 10.1016/j.jim.2012.03.007
- Bologna G, Lanuti P, D'Ambrosio P, Tonucci L, Pierdomenico L, D'Emilio C, Celli N, Marchisio M, d'Alessandro N, Santavenere E, Bressan M, Miscia S. Water-soluble platinum phthalocyanines as potential antitumor agents. Biometals. 27(3):575-89 (2014) ISSN: 0966-0844). IF: 3.284. 10.1007/s10534-014-9730-y
- Veronese A, Pepe F, Chiacchia J, Pagotto S, Lanuti P, Veschi S, Di Marco M, D'Argenio A, Innocenti I, Vannata B, Autore F, Marchisio M, Wernicke D, Verginelli F, Leone G, Rassenti LZ, Kipps TJ, Mariani-Costantini R, Laurenti L, Croce CM, Visone R. Allele-specific loss and transcription of the miR-15a/16-1 cluster in chronic lymphocytic leukemia. Leukemia. 29(1):86-95. (2015) ISSN: 0887-6924. IF: 12.104. 10.1038/leu.2014.139
- Lachmann R, Lanuti P, Miscia S. OMIP-011: Characterization of circulating endothelial cells (CECs) in peripheral blood. Cytometry A. 81(7):549-51. (2012) ISSN: 1552-4930. (http://www.ncbi.nlm.nih.gov/pubmed/22648996). IF: 3.753. 10.1002/cyto.a.22071
- Lanuti P, Rotta G, Almici C, Avvisati G, Budillon A, Doretto P, Malara N, Marini M, Neva A, Simeone P, Di Gennaro E, Leone A, Falda A, Tozzoli R, Gregorj C, Di Cerbo M, Trunzo V, Mollace V, Marchisio M, Miscia S. Endothelial progenitor cells, defined by the simultaneous surface expression of VEGFR2 and CD133, are not detectable in healthy peripheral and cord blood. Cytometry A. doi: 10.1002/cyto.a.22730. (2016) IF:2.928. 10.1002/cyto.a.22730
- Codagnone M, Recchiuti A, Lanuti P, Pierdomenico AM, Cianci E, Patruno S, Mari VC, Simiele F, Di Tomo P, Pandolfi A, Romano M. Lipoxin A4 stimulates endothelial miR-126-5p expression and its transfer via microvesicles. FASEB J. 2017 Jan 18. pii: fj.201600952R. doi: 10.1096/fj.201600952R (2017). IF:5.229. 10.1096/fj.201600952R
- Di Tomo P, Lanuti P, Di Pietro N, Baldassarre MPA, Marchisio M, Pandolfi A, Consoli A, Formoso G. Liraglutide mitigates TNF-α induced pro-atherogenic changes and microvesicle release in HUVEC from diabetic women. Diabetes Metab Res Rev. 2017 Nov;33(8). doi: 10.1002/dmrr.2925. Epub 2017 Sep 6. IF: 3.263. 10.1002/dmrr.2925
- Verginelli F, Perconti S, Vespa S, Schiavi F, Prasat SC, Lanuti P, Cama A, Tramontana L, Esposito DL, Guarnieri S, Sheu A, Pantalone MR, Florio R, Morgano A, Rossi C, Bologna G, Marchisio M, D'Argenio A, Taschin E, Visone R, Opocher G, Veronese A, Paties CT, Rajasekhar VK, Söderberg-Nauclér C, Sanna M, Lotti LV, Mariani-Costantini R. Paragangliomas arise through an autonomous vasculo-angio-neurogenic program inhibited by imatinib. Acta Neuropathol. 2018 Jan 5. doi: 10.1007/s00401-017-1799-2. IF: 12.213. 10.1007/s00401-018-1811-5
- Lanuti P, Simeone P, Rotta G, Almici C, Avvisati G, Azzaro R, Bologna G, Budillon A, Di Cerbo M, Di Gennaro E, Di Martino ML, Diodato A, Doretto P, Ercolino E, Falda A, Gregorj C, Leone A, Losa F, Malara N, Marini M, Mastroroberto P, Mollace V, Morelli M, Muggianu E, Musolino G, Neva A, Pierdomenico L, Pinna S, Piovani G, Roca MS, Russo D, Scotti L, Tirindelli MC, Trunzo V, Venturella R, Vitagliano C, Zullo F, Marchisio M, Miscia S. A standardized flow cytometry network study for the assessment of circulating endothelial cell physiological ranges. Sci Rep. 2018. 11;8(1):5823. doi: 10.1038/s41598-018-24234-0. IF: 4.259. 10.1038/s41598-018-24234-0
- Pieragostino D, Lanuti P, Cicalini I, Cufaro MC, Ciccocioppo F, Ronci M, Simeone P, Onofrj M, van der Pol E, Fontana A, Marchisio M, Del Boccio P. Proteomics characterization of extracellular vesicles sorted by flow cytometry reveals a disease-specific molecular cross-talk from cerebrospinal fluid and tears in multiple sclerosis. J Proteomics. 2019 Jun 3;204:103403. doi: 10.1016/j.jprot.2019.103403. IF: 3.537. 10.1016/j.jprot.2019.103403
- Ciardiello C, Leone A, Lanuti P, Roca MS, Moccia T, Minciacchi VR, Minopoli M, Gigantino V, De Cecio R, Rippa M, Petti L, Capone F, Vitagliano C, Milone MR, Pucci B, Lombardi R, Iannelli F, Di Gennaro E, Bruzzese F, Marchisio M, Carriero MV, Di Vizio D, Budillon A. Large oncosomes overexpressing integrin alpha-V promote prostate cancer adhesion and invasion via AKT activation. J Exp Clin Cancer Res. 2019 Jul 18;38(1):317. doi: 10.1186/s13046-019-1317-6. IF: 5.646. 10.1186/s13046-019-1317-6
- Sorrentino C, Yin Z, Ciummo S, Lanuti P, Lu LF, Marchisio M, Bellone M, Di Carlo E. Targeting Interleukin(IL)-30/IL-27p28 signaling in cancer stem-like cells and host environment synergistically inhibits prostate cancer growth and improves survival. J Immunother Cancer. 2019 Jul 31;7(1):201. doi: 10.1186/s40425-019-0668-z. IF: 8.676. 10.1186/s40425-019-0668-z
- Rossi C, Cicalini I, Cufaro MC, Agnifili L, Mastropasqua L, Lanuti P, Marchisio M, De Laurenzi V, Del Boccio P, Pieragostino D. Multi-Omics Approach for Studying Tears in Treatment-Naïve Glaucoma Patients. Int J Mol Sci. 2019 Aug 18;20(16). pii: E4029. doi: 10.3390/ijms20164029. IF: 4.183. 10.3390/ijms20164029
- Cossarizza A,[… ], Lanuti P,[…]. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur J Immunol. 2019 Oct;49(10):1457-1973. doi: 10.1002/eji.201970107. IF: 5.179. 10.1002/eji.201970107
- Brocco D, Lanuti P, Simeone P, Bologna G, Pieragostino D, Cufaro MC, Graziano V, Peri M, Di Marino P, De Tursi M, Grassadonia A, Rapposelli IG, Pierdomenico L, Ercolino E, Ciccocioppo F, Del Boccio P, Marchisio M, Natoli C, Miscia S, Tinari N. Circulating Cancer Stem Cell-Derived Extracellular Vesicles as a Novel Biomarker for Clinical Outcome Evaluation. J Oncol. 2019 Nov 18;2019:5879616. IF: 2.600. 10.1155/2019/5879616


