Paraganglioma Biobank
Paragangliomas: overview
Paragangliomas (PGLs) are rare hypervascular tumors of the paraganglia, organelles connected with the autonomic branches of the cranial (head and neck) and thoraco-lumbar nerves (Pacak and Wimalawansa, 2015). PGLs grow slowly, but are highly infiltrating, may unpredictably metastasize and are refractory to chemo/radiotherapy. Head and neck PGLs (~ 20% of all PGLs) are of particular concern, as they spread along the regional neurovascular structures towards the skull base, may insinuate intracranially and may compress the brainstem.
The organoid structure is constituted by dysmorphic variants of the vascular and neural tissues found in normal paraganglia. The cardinal feature shared by PGLs and paraganglia is the integration of a neurosecretory network, consisting in nests or cords of glia-bound neuroepithelial cells (“zellballens”), with an angiomatous vasculature.
About 40% of PGL patients carry a predisposing mutation in one of at least 18 cancer-related nuclear genes, including those that encode the 4 subunits of the SDH (complex II) enzyme of the mitochondrial (mt) electron transport chain, SDHA/B/C/D and the assembly factor SDHAF2 (collectively named SDHx genes), essential for energy production under aerobiosis.
The CAST paraganglioma research biobank
The paraganglioma research biobank at CAST has been collected between 2009 and 2019, thanks to collaborations with the Otology & Skull Base Surgery team of Gruppo Otologico operating at the Casa di Cura Piacenza, Piacenza Italy, led by Professor Mario Sanna, and with the Service of Anatomic Pathology, Guglielmo da Saliceto Hospital, Piacenza. The biological material is coded and has been collected with informed patient consent, within the framework of two research projects supported by the Italian Association for Cancer Research (AIRC), that were approved by the competent bioethical committees. Coded samples can be requested on a collaborative basis, provided that the use is in agreement with the aims of the original research projects, that were to promote knowledge on the etiopathogenesis of the disease and contribute to the discovery of new treatments.
Researchers interested in the samples can directly contact Dr. Fabio Verginelli, head of the biobank (verginelli@unich.it).
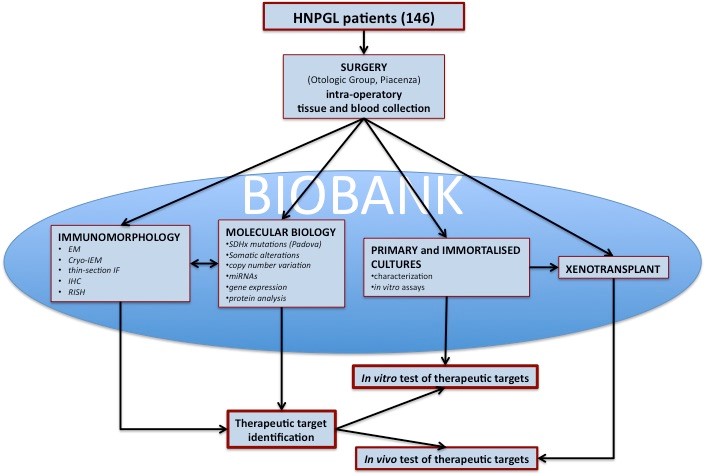
Figure 1. Flowchart of the PGL biobank. The biological material sampled in the operating room is preserved to allow morphological, genomic, proteomic analyses and the development of cellular and animal models. Data analyses and availability of in vitro and in vivo models allows to carry out tests for the identification of therapeutic targets. HNPGL = head and neck paraganglioma.
Biobank samples
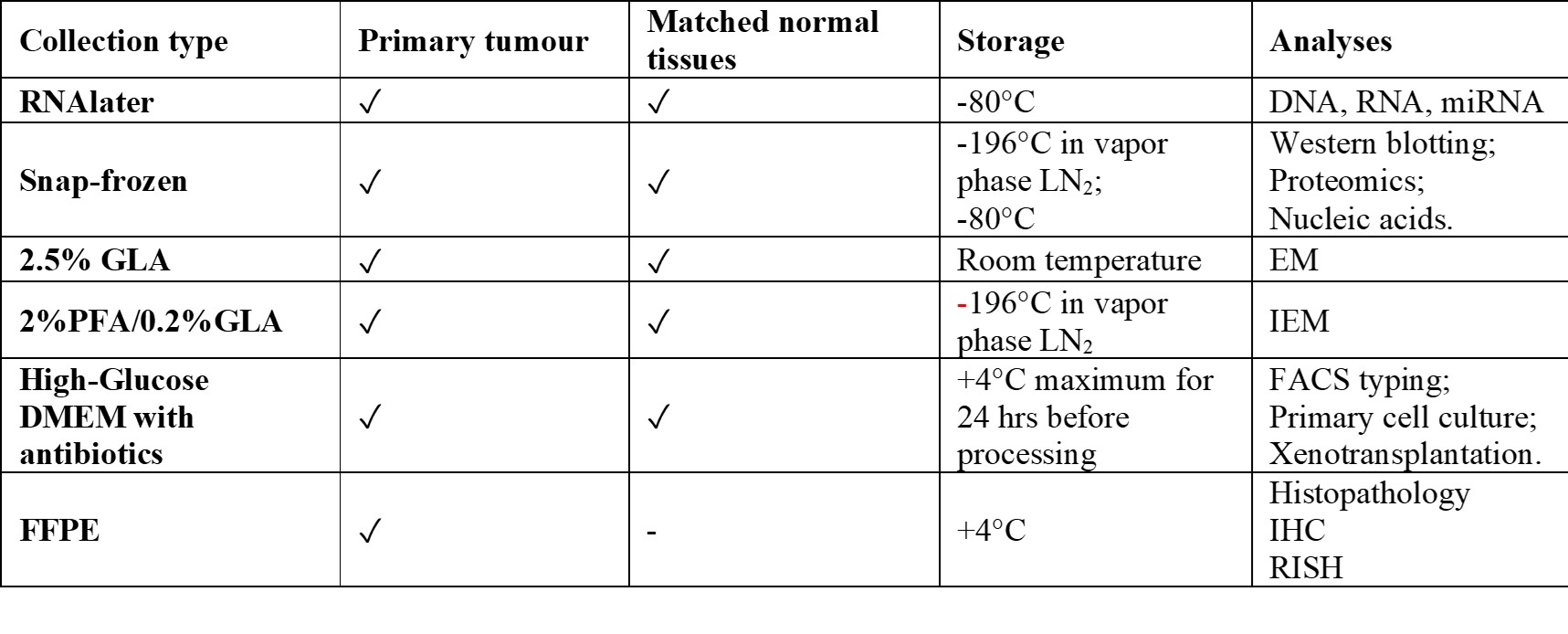
As shown in figure 1, for each of 146 consenting patients, we obtained primary tumor matched with blood and any normal tissue (nerves, vessels, skin, fibromuscular, connective tissue and bone) removed from the neck and base of the skull and for some cases adipose tissue from abdomen. Moreover, from some cases under trans-labirinthine vestibular surgery we sampled the Jacobson’s nerve that could represent a normal tissue of origin of head and neck PGL (HNPGL). Biobank samples for each case are collected, preserved and analyzed as follows: GLA: glutaraldehyde; PFA: paraformaldehyde; EM: electron microscopy; IEM: immuno electron microscopy; IHC: immunohistochemistry; RISH: rapid in situ hybridization All 146 prospectively collected PGLs, together with a retrospective series of about 200 cases, are available in formalin-fixed, paraffin-embedded blocks. The biobank includes also 100 PGL-derived xenografts (snap frozen, FFPE and 2.5% GLA for electron microscopy). Furthermore, 39 primary PGL cell cultures obtained from fresh collected samples are maintained in vapor phase liquid nitrogen freezer. Four primary cultures were immortalized by retroviral-mediated transduction of full-length hTERT and/or simian virus 40 (SV40) large-tumour (LT) antigen.READ MORE
Features of the paraganglioma series
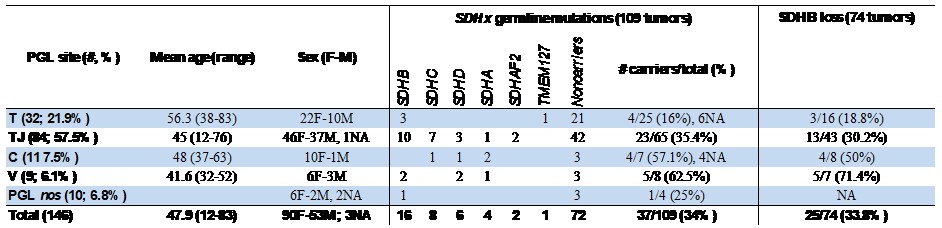
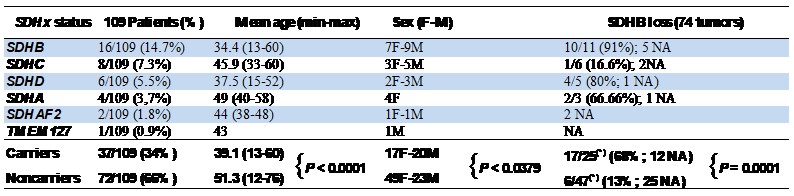
The biological samples in the research biobank are from 146 head and neck PGL patients recruited between 2009 and 2019 including 84 tympano-jugular, 32 tympanic, 11 carotid body, 9 vagal and other 10 cases are under classification and temporarily listed as PGL "not otherwise specified" (nos). One case had lymph node metastases, one case had two independent tumors (carotid body and vagal), 9 patients presented recurrency. For all cases preoperative urinary and plasma catecholamines were negative, in agreement with the mostly parasympathetic (non secretory) origin of head and neck paragangliomas. Only two patients reported a family history of paraganglioma. The overall mean age at surgery of the patients is 48 (range: 12-83) years old. Tympanic PGLs are associated in our series with higher age of the patients (mean: 56.3 yo), significantly different from the age of the patients with tympano-jugular (mean: 45 yo, P= 0,0002), vagal (mean: 41.6 yo, P=0,0025) and carotid body (mean: 48 yo, P=0.038) PGLs (Table 1). Table 1: Sites of tumor origin, age and sex of the 146 PGLs of the series (column at left). SDHx germline mutation status in a subset of 109 PGL (column at center). SDHB immunohistochemistry (IHC) in a subset of 74 PGL of the series (column at right). The comparatively high frequencies of tympanic and tympano-jugular PGLs reflects recruitment at a specialized skull base surgery center. NA: not available. Up to now, 109 out of 146 cases are characterized for germline point mutations and large deletions/rearrangements in the SDHA, SDHB, SDHC, SDHD, SDHAF2 and TMEM27 genes, in collaboration with Dr. Schiavi at Familial Cancer and Oncoendocrinology Unit - Veneto Institute of Oncology IRCCS. Somatic mutations and loss of heterozygosity analyses are ongoing on all cases. SDHB is affected by germline mutations in 16 cases (14,7%), followed in order by SDHC (8 cases, 7.3%), SDHD (6 cases, 5.5%), SDHA (4 cases, 3.9%), SDHAF2 (2, 1,8%) and TMEM127 (1 case, 0.9%), with an overall percentage of carriers equal to 34% compared to 66% of noncarriers (Table 1 and Table 2). Table 2. SDHx status, age and sex of 109 patients screened for mutations and tumor-associated SDHB protein loss in a subset of 74 mutation carriers and noncarriers.READ MORE
Cama A, Verginelli F, Lotti LV, Napolitano F, Morgano A, D’Orazio A, Vacca M, Perconti S, Pepe F, Romani F, Sanna M, Mariani-Costantini R (2013) Integrative genetic, epigenetic and pathological analysis of paraganglioma reveals complex dysregulation of NOTCH signaling. Acta Neuropathol 126:575–594.
Gill AJ, Benn DE, Chou A, Clarkson A, Muljono A, Meyer- Rochow GY et al (2010) Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma- pheochromocytoma syndromes. Hum Pathol 41:805–814.
Kantorovich V, Pacak K (2018). New insights on the pathogenesis of paraganglioma and pheochromocytoma. F1000Research 7:Faculty Rev-1500.
Lotti LV, Vespa S, Pantalone MR, Perconti S, Esposito DL, Visone R, Veronese A, Paties CT, Sanna M, Verginelli F, Nauclér CS, Mariani-Costantini R (2019) A developmental perspective on paragangliar tumorigenesis. Cancers (Basel) 11(3):273.
Pacak K, Wimalawansa SJ (2015) Pheochromocytoma and paraganglioma. Endocr Pract 21:406–412.
Taieb D, Kaliski A, Boedeker CC, Martucci V, Fojo T, Adler JR Jr et al (2014) Current approaches and recent developments in the management of head and neck paragangliomas. Endocr Rev 35:795–819.
Verginelli F, Perconti S, Vespa S, Schiavi F, Prasad SC, Lanuti P, Cama A, Tramontana L, Esposito DL, Guarnieri S, Lotti LV, Mariani-Costantini R (2018) Paragangliomas arise through an autonomous vasculo-angio-neurogenic program inhibited by imatinib. Acta Neuropathol 135:779-798.
