Cytomegalovirus in human tumorigenesis
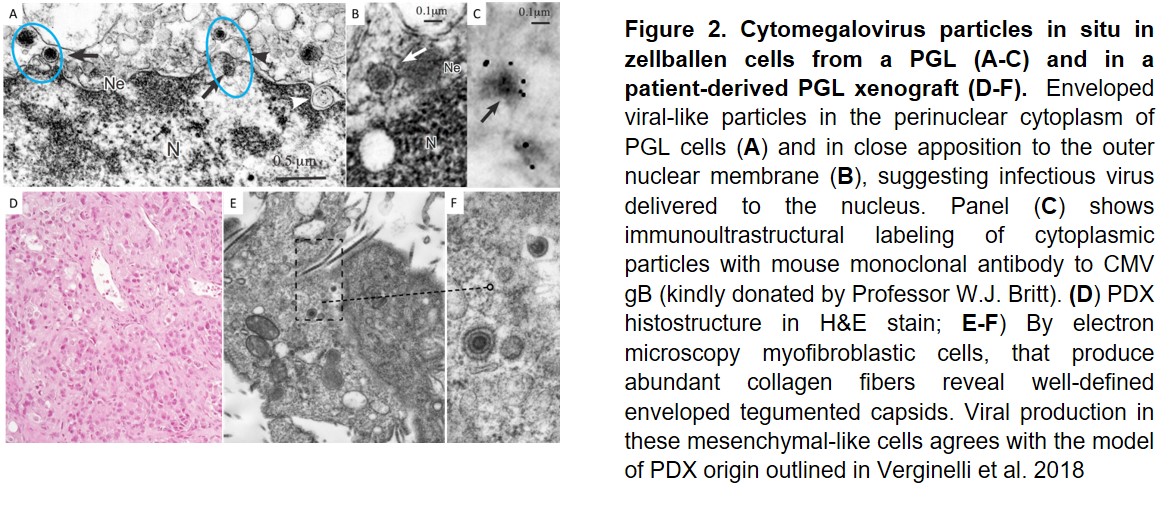
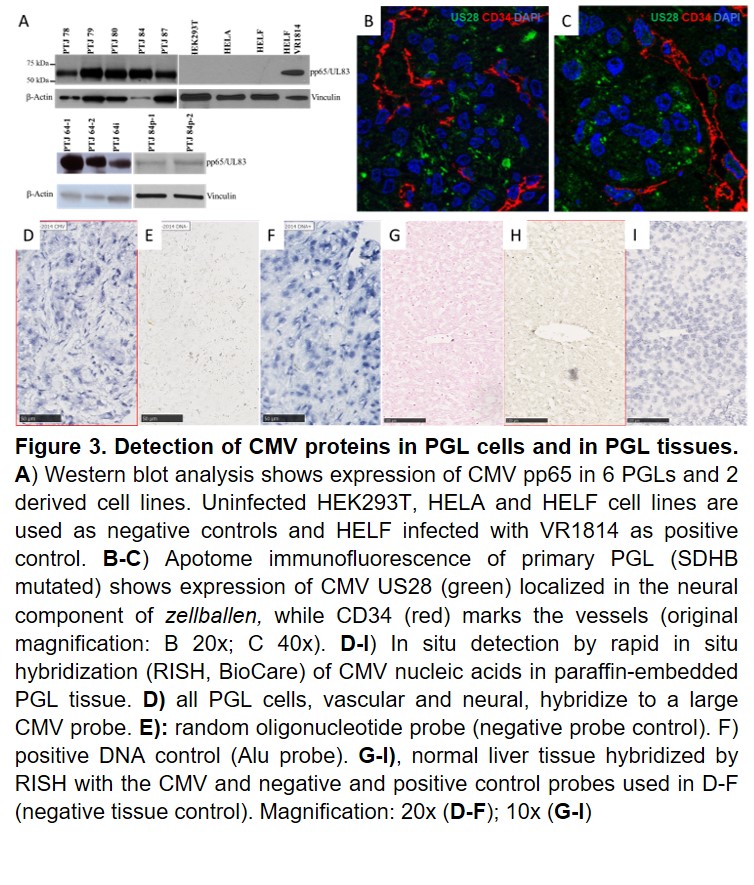
Our research activity builds on our original data indicating that paragangliomas/pheochromocytomas (PGLs), rare neural crest-derived tumors thought to be mainly due to genetic risk factors, develop through a deregulated neural crest vasculo-angio-neurogenic developmental program associated with infection of PGL stem-like cells with a human cytomegalovirus (CMV)-like virus, which causes metabolic rewiring, pseudohypoxia signaling, and genetic and epigenetic modifications promoting proliferation/differentiation along an HIF-2A-related EMT program. Our work relies on our new original methods to isolate tumor-derived CMV from tumor tissue and urine of patients. We demonstrated that CMV-like virus particles can be detected and purified from PGLs of different anatomic sites and genetic background and that these particles can infect other cells, inducing alterations different from those caused by a laboratory strain of CMV at similar concentrations. In collaboration with colleagues at the Karolinska Institutet in Stockholm (Prof. Cecilia Soderberg Nauclèr; Dr. Igor Adameyko, Dr. Ninib Baryawno) we are attempting to dissect the developmental pathways aberrantly activated by viral infection in PGLs in relation to data on normal paragangliar development.
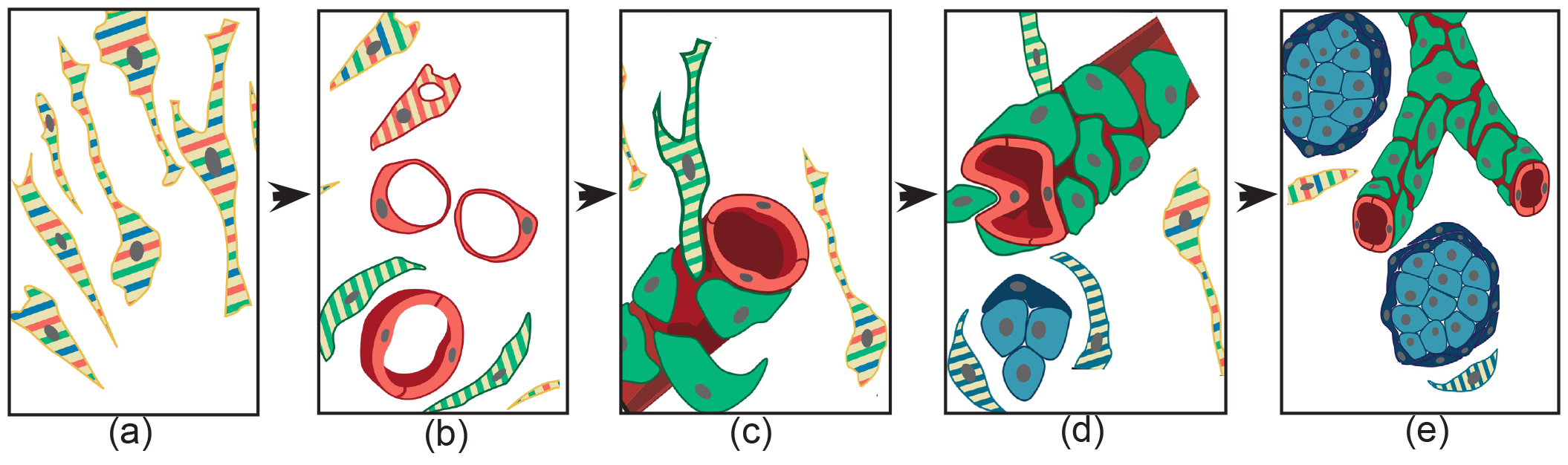
Figure 1. Schematic representation of the histogenesis of paraganglioma, based on patient- and cell-derived xenograft models. The thin elongated stem-like cells (a), stabilized and expanded in paraganglioma cell cultures, co-express (stripes) multipotent stem/progenitor cell markers. In vivo, (b) these cells grow on autonomously synthesized extracellular matrix, develop intracytoplasmic vacuoles of increasing size (cells with red stripes only), and coalesce (uniformly red cells), giving rise to endothelial tubes (vasculogenesis via cytoplasmic hollowing). The endothelium then recruits adjacent stem-like cells (cells with green stripes only), which, after contact with the abluminal endothelial membranes (c), differentiate into mural cells (uniformly green). (d) The image depicts two consequences of mural stabilization. First, mural impingement results in endothelial intussusceptions, which give rise to Y-shaped vascular ramifications (intussusceptive angiogenesis). Secondly, vascular stabilization results in perivascular deposition of collagen IV, which supports the development of cell clusters with neural phenotype (cells with blue stripes only, then uniformly blue). As shown in (e), these clusters develop into “zellballen”-like neuroepithelial nests (uniform dark blue). Notably, while mesenchymal paraganglioma stem-like cells have normal mitochondria, paraganglioma tissue organization is associated with increasing mitochondrial dysfunction (swelling and loss of membrane potential), culminating in the neuroepithelial component. Original art by Giulio Pandolfelli, from: Lotti LV, et al. A Developmental Perspective on Paragangliar Tumorigenesis. Cancers (Basel) 2019 Feb 26;11(3). pii: E273. doi: 10.3390/cancers11030273.
READ MORE
Hypothesis
We hypothesize that genetically predisposed subjects develop PGL because pre-existing defects in metabolism and hypoxia responses facilitate tumorigenic infection of PGL stem-like cells expressing CMV receptors linked to mesenchymal (vascular/perivascular) and neural (glial/neuronal) differentiation, which results in the build-up of an organoid tumor mass through mechanisms that essentially recapitulate, although aberrantly, an inherent developmental program that is also implicated in postnatal adaptation to hypoxic conditions and in tissue repair after damage.READ MORE
Aims
We aim at the genomic and functional characterization of the PGL CMV-like virus, which is a difficult task due to the high grade of intrahost variability of these viruses that chronically infect the cancer microenvironment. We intend to define the role of viral infection and of other triggering factors on the metabolic and epigenetic control of neural crest differentiation. Our new concept of PGL origin needs to be substantiated by identifying host, viral and niche factors precipitating and driving neural crest differentiation, CMV replication and enhanced proliferation of PGL cells. We also aim at validating in preclinical models PGL treatments targeting CMV and neural crest development.READ MORE
Experimental Approach
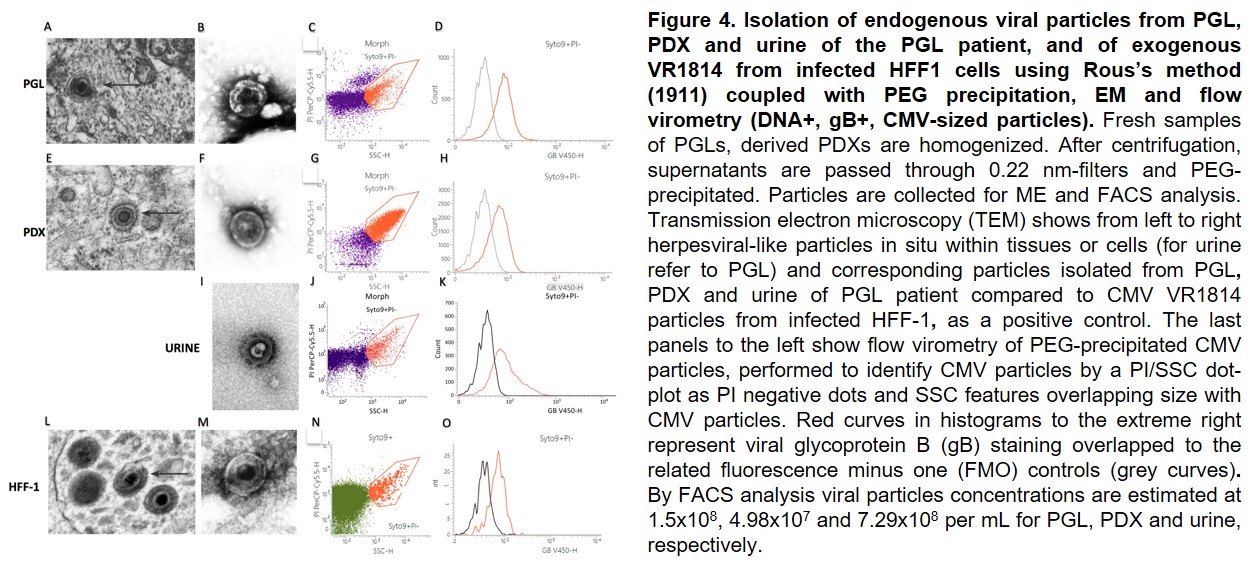
CMV particles are isolated from PGL/PDX tissues, urine of patients and PDX mice using Rous's protocol coupled with PEG precipitation and characterized by FACS and EM. Isolated particles are used to: define CMV heterogeneity using Nanopore Sequencing; infect/superinfect normal or PGL cultures in comparison with lab CMV strains. Infected cells are used to assess cytopathic, transcriptional (single-cell sequencing) and transforming effects. 2D transcriptomics integrated with single-cell sequencing will be used to identify the PGL cell of origin and whether infection hijacks neural crest developmental programs utilizing receptors on PGL stem-like cells. Interactions between CMV and neural crest differentiation are assessed by comparing PGL/PDX-derived data to data from physiologic paragangliar development. Drugs targeting the CMV-infected PGL cells directly or through microenvironmental interactions are identified using patient-derived in vitro and in vivo PGL models.READ MORE
Impact on Cancer
We propose PGL as a new model of human carcinogenesis at the interface between genetic variation, development and predisposition to oncogenic viral infection. This model could apply to other cancers where CMV-like viruses were controversially reported, and that we are also currently investigating in parallel with PGL.
- Neumann HPH, et al. Pheochromocytoma and Paraganglioma. The New England journal of medicine. 2019;381(6):552-65.
- Cama, A., et al., Integrative genetic, epigenetic and pathological analysis of paraganglioma reveals complex dysregulation of NOTCH signaling. Acta Neuropathol 2013 126(4): 575-94.
- Verginelli F, et al. Paragangliomas arise through an autonomous vasculo-angio-neurogenic program inhibited by imatinib. Acta Neuropathol 2018; 135:779-798. doi: 10.1007/s00401-017-1799-2.
- Lotti LV, et al. A Developmental Perspective on Paragangliar Tumorigenesis. Cancers (Basel) 2019 Feb 26;11(3). pii: E273. doi: 10.3390/cancers11030273.
- Heine U, Kondratick J, Ablashi DV, Armstrong GR, Dalton AJ. Ultrastructural and biological properties of a cytomegalovirus rescued from a human paraganglioma. Cancer Res 1971;31(5):542-9.
- Geder L, Kreider J, Rapp F. Human cells transformed in vitro by human cytomegalovirus: tumorigenicity in athymic nude mice. J Natl Cancer Inst 1977;58(4):1003-9.
- Herbein G. The Human Cytomegalovirus, from Oncomodulation to Oncogenesis. Viruses. 2018;10(8).
- Baryawno N, et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J Clin Invest 2011, 121(10): 4043-4055.
- Rous P. A Sarcoma of the Fowl Transmissible by an Agent Separable from the Tumor Cells. The Journal of Experimental Medicine. 1911;13(4):397-411.
- Warburg O. On the origin of cancer cells. Science, 1956. 123(3191): 309-14.
- Seyfried TN. Cancer as a mitochondrial metabolic disease. Front Cell Dev Biol, 2015. 3: 43. 10.1016/j.ymben.2016.11.005
- Furlan A, et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science, 2017. 357(6346).





