Molecular pathology
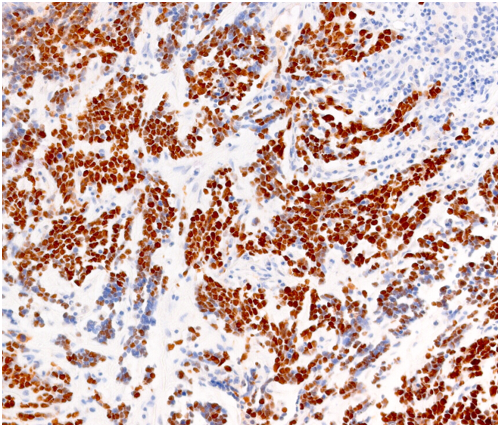
STAINS: Hematoxylin & Eosin, Special stains, Immunohistochemical stains
INFO Service
CAST’s Histopathology Lab provides University researchers the resources for processing and evaluation of tissue specimens derived from experimental pathology models and clinical specimens. In the Histopathology Lab, particular emphasis is given to the pathological phenotyping of tissue specimens taken from skin, lung, liver, pancreas, spleen, kidney, urinary bladder, breast, male and female reproductive systems, thyroid gland, thymus gland, and brain. To pursue this mission, the Histopathology Lab has established a comprehensive histopathological pipeline that provides technical assistance and training for:
- Tissue processing and sample preparation
- Tissue fixation and embedding in paraffin and OCT blocks
- Preparation of paraffin-embedded sections and cryosections
- Tissue Microarrays (TMA) construction
- H&E / special stains
- Immunohistochemistry (IHC)
- Immunofluorescence (IF)
IHC is a specialized section of the Histopathology Lab and the technique involves the identification of specific targets (antigens) within cells and tissues. This involves the use of antibodies that are directed towards the specific targets of interest. The resulting antigen/antibody reaction is then visualized by tagging with a permanent label (chromogen or fluorophore). The presence of the antigen of interest can then be seen by microscopy. Through multiple light microscopes, the Histopathology Lab staff can assist University researchers in taking slide pictures in multiple formats (i.e., TIFF/JPEG/PNG/BMP) suitable for any publication purposes.
PROCESSING & SECTIONING: Sectioning of paraffin and OCT embedded tissue blocks
DATA ANALYSIS: Digital microscope acquisitions and data documentation by certified Pathologists
INFO Access
The Histopathology Lab staff is set to provide basic and advanced training for the University researchers on the correct use of the histopathological techniques and of the immunohistochemical staining procedures. For this purpose, University researchers are required to attend practical training seminars held by the Histopathology Lab staff before being allowed to use any specific Lab instrument or staining procedure.
Leica ASP 300 Tissue Processor
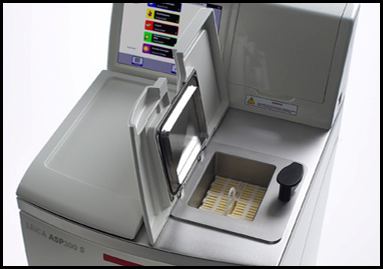
The Leica ASP300 is a fully enclosed, stand-alone processor for paraffin infiltration of tissues. It is designed for routine and research histopathology of up to 300 cassettes. It is provided by a Reagent Management System (RMS) that allows customizing the use of reagents by days, runs or cassettes, and a quick-start feature for commonly used programs. The tissue processor allows four types of gentle agitation, temperature control, and optical and maintenance-free level sensors to ensure consistent optimal sample quality. All important data sets from each process are logged in a data file and can be printed or downloaded to a disk. The tissue processor is able to detect power failures or operational errors such as missing or under-filled reagent bottles and either corrects the problem automatically or displays a recommended solution to safeguard the samples. Acquisition year: 2003READ MORE
Leica EG-1160 Embedding Center

The Leica EG 1160 Tissue Embedding Center features a three-liter paraffin reservoir and has a scratch-resistant heated working surface with a paraffin drain system. Acquisition year: 2003READ MORE
It has two heated removable paraffin waste trays, a removable and interchangeable cassette and mold warmer, and a large peltier cooling plate. Temperatures are adjustable on the heating modules between 45 °C and 70 °C, and there is a programmable on/off timer. Capacity is approximately 100 cassettes and/or molds.
Leica RM2125 RT Microtome

The Leica RM2125 RT rotary microtome features a two-step trimming (50 µm coarse, 10 µm fine) and specimen retraction (ON/Off approx. 20 µm) functions. The manual sectioning via a counter-balanced handwheel makes it specifically suitable for paraffin sectioning applications for both routine and special applications. Section thickness setting range from 0,5 to 60 µm. Acquisition year: 2003READ MORE
Leica CM1850 Cryostat

The Leica CM1850 is a versatile cryostat with an optimized cooling system, rapid specimen freezing, and smooth specimen orientation for the high-quality sectioning demanded for both routine and research histology specimens. Section thickness setting range from 1 to 60 µm (1 µm increments from 1 to 10 µm; 2 µm increments from 10 to 20 µm; 5 µm increments from 20 to 60 µm). The maximum specimen size is 55 x 55 mm. The temperature setting range from 0°C to -35°C. Acquisition year: 2003READ MORE
Dako Autostainer E-172566

The Dako Autostainer E-172566 System is an automated slide processing open system compatible with all reagents used for the staining of paraffin-embedded and frozen tissue sections, cytospins, cell smears, and fine-needle aspirates. It can handle 64 different reagents (15 mL/reagent vial). It dispenses reagent volumes from 100 to 600 µL. This system is designed to automate manual staining methods routinely used in immunohistochemistry and cytochemistry, enabling the transfer of established protocols from the bench to the Autostainer. Alteration of standard reagents or experimental conditions may not be required. It is provided of 4 racks, each of them can hold 12 microscope slides in a horizontal position. Glass slide dimensions supported are 25mm x 75mm x 1mm. Acquisition year: 2003READ MORE
Manual Tissue Arrayer MTA-1

The manual arrayer allows for the construction of tissue microarrays (TMA). It is a manually operated high-precision arraying instrument that can create arrays until 500 samples per recipient block. The tissue arrayer is provided with two pairs of punches with stylets, in size of 0.6 mm, 1.0 mm, 1.5 mm, and 2.0 mm diameter. The speed (cores/hour) in manual assembling of the TMA block is 30-70. The max donor block height allowed is 3 cm. Acquisition year: 2005READ MORE
Note: the Histopathology Lab is also provided with other instruments (i.e., base-sledge microtome, tissue floatation water bath, Leica ST5010 Autostainer XL, convection oven, microwave oven, pressure cooker) and histology consumables (i.e., slides, paraffin, microtome blades, cassettes, reagents & solutions for routine, special, and IHC stains, mounting media & adhesives, frozen section media).
Bomba M, Granzotto A, Castelli V, Onofrj M, Lattanzio R, Cimini A, Sensi SL. Exenatide reverts the high-fat-diet-induced impairment of BDNF signaling and inflammatory response in an animal model of Alzheimer's disease. Journal of Alzheimer's Disease. 2019:1-18. doi: 10.3233/JAD-190237
Giansanti F, Capone E, Ponziani S, Piccolo E, Gentile R, Lamolinara A, Di Campli A, Sallese M, Iacobelli V, Cimini A, De Laurenzi V, Lattanzio R, Piantelli M, Ippoliti R, Sala G, Iacobelli S. Secreted Gal-3BP is a novel promising target for non-internalizing Antibody-Drug Conjugates. Journal of Controlled Release. 2019; 294:176-184. doi: 10.1016/j.jconrel.2018.12.018
Lattanzio R, Iezzi M, Sala G, Tinari N, Falasca M, Alberti S, Buglioni S, Mottolese M, Perracchio L, Natali PG, Piantelli M. PLC-gamma-1 phosphorylation status is prognostic of metastatic risk in patients with early-stage Luminal-A and -B breast cancer subtypes. BMC Cancer. 2019;19. doi:10.1186/s12885-019-5949-x
Guerra E, Trerotola M, Tripaldi R, Aloisi AL, Simeone P, Sacchetti A, Relli V, D'amore A, La Sorda R, Lattanzio R, Piantelli M, Alberti S. Trop-2 induces tumor growth through Akt and determines sensitivity to Akt inhibitors. Clinical Cancer Research. 2016;22:4197-4205. doi: 10.1158/1078-0432.CCR-15-1701
De Cola A, Volpe S, Budani MC, Ferracin M, Lattanzio R, Turdo A, D'agostino, D, Capone E, Stassi G, Todaro M, Di Ilio C, Sala G, Piantelli M, Negrini M, Veronese, A, De Laurenzi Vincenzo. Mir-205-5p-Mediated Downregulation Of Erbb/HER Receptors In Breast Cancer Stem Cells Results In Targeted Therapy Resistance. Cell Death & Disease. 2015;6:e1823. doi: 10.1038/cddis.2015.192
Simeone P, Trerotola M, Urbanella A, Lattanzio R, Ciavardelli D, Di Giuseppe F, Eleuterio E, Sulpizio M, Eusebi V, Pession A, Piantelli M, Alberti S. A unique four-hub protein cluster associates to glioblastoma progression. Plos One. 2014;9.ISSN: 1932-6203. doi:10.1371/journal.pone.0103030
Esposito DL, Aru F, Lattanzio R, Morgano A, Abbondanza M, Malekzadeh R, Bishehsari F, Valanzano R, Russo A, Piantelli M, Moschetta A, Lotti LV, Mariani-Costantini R. The insulin receptor substrate 1 (IRS1) in intestinal epithelial differentiation and in colorectal cancer. Plos One. 2012;7:1-14. doi: 10.1371/journal.pone.0036190
 Rossano LattanzioAssociate Professor of |
||


