Functional Biotechnologies
Our area of interest falls in the study of the cellular responses to the micro- and macro-environment, in view of translational applications in the field of biomedicine. In particular, various cell models (skeletal and cardiac muscle, neuronal cells, peripheral blood lymphocytes, adult stem cells) are used to investigate the responses to chemical (growth factors, hormones, drugs) and physical (low frequency electromagnetic radiation, microgravity) agents, and the interactions between cells and biocompatible materials.
The behaviour of the cell models studied is analysed focusing on signal transduction mediated by intracellular calcium variation, electrical membrane activity and oxidative/metabolic state, in which mitochondrial activity plays a major role.
The techniques used are based on conventional and confocal fluorescence and patch-clamp techniques.
Characterization of the role of the Growth Associated Protein 43 (GAP43) in muscle cells
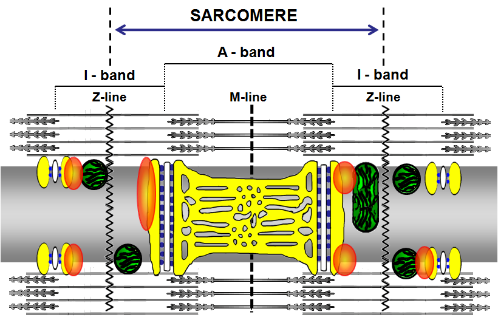
A model proposing GAP43 positioning in skeletal muscle. In the proposed model the fiber is seen along its longitudinal axis. The sarcoplasmic reticulum is reproduced in yellow, while mitochondria on both sides of the Z–lines, are represented in green. The white structures represent the T-tubules and the blue spots are ryanodine receptors. Orange blobs represent GAP43. Guarnieri et al, 2013, PLoS ONE 8(1): e53267. doi:10.1371/journal.pone.0053267 In recent years, a casual event, such as the use of antibodies against specific markers in neuromuscular co-cultures, has attracted our attention to the presence of Growth Associated Protein 43 (GAP43), a neuronal marker, in skeletal muscle cells. A study was thus developed which defined in mouse and human skeletal myocells the presence of this protein, with different localizations according to undifferentiated or mature phenotype. In the latter, the protein is arranged regularly between the mitochondria and the calcium release units, suggesting a specific functional role (Guarnieri et al, PlosOne 2013). The presence of the protein also in skeletal muscle of fish and amphibians supports the strategic role that the protein plays in muscle (Caprara et al, Eur J Histochem 2014). The use of transgenic knockout mice for GAP-43 allowed to verify the hypothesis that, in skeletal muscle, GAP43 interacts with calmodulin to indirectly modulate the activity of the RyR and DHPR channels. This may influence the dynamics of Ca2+ homeostasis and, consequently, all the downstream processes, including excitation-contraction coupling, gene expression, cell metabolism (Caprara et al, Front Physiol 2016).READ MORE
Analysis of cell homeostasis following environmental challenges (electromagnetic fields and gravity)
Not less involving have been studies focused on the effects of extremely low frequencies electromagnetic fields (ELF-EMF) on various animal and cell models. A multi-year project, funded by the Italian Ministry of the Environment and Protection of the Territory, allowed a multidisciplinary research that addressed both technological problems (such as the development and standardization of systems for generating electromagnetic fields and tools for checking their efficiency, Farina et al, Progr Electromagn Res-B 2010) and biological issues, such as the effects of ELF-EMF "in vitro" (on prokaryotic and eukaryotic cells) and “in vivo” (on young and elderly rats, and transgenic mice predisposed to mammary tumors). The first results highlighted that exposure to ELF-EMF induces oxidative stress (Mariggiò, Amicarelli et al, In Biological Effects of Electromagnetic Fields 2006; Falone et al , Int J Biochem Cell Biol 2008; Morabito et al, Free Radic Biol Med 2010; Morabito et al, Cell Physiol Biochem, 2010). The field intensities used (0.1-1.0 mT) triggered adaptative mechanisms both at the cellular level (through compensation systems involving intracellular Ca2+ signalling and cellular redox systems) and at the organismal level (Mariggiò, Amicarelli et al, Mariggiò, Morabito et al, Iezzi et al, In Biological Effects of Electromagnetic Fields 2006; Falone et al, Int J Biochem Cell Biol 2008; Morabito et al, Free Radic Biol Med 2010; Morabito et al, Cell Physiol Biochem, 2010 ; Morabito et al, BiomedResInt. 2017). Prokaryotic cells seem to be more susceptible to electromagnetic exposures in terms of morphological changes and transcriptional signatures (Cellini et al, Bioelectromagnetics 2008). As part of a project concerning the study of the intercellular relationships within the nervous system, a human astrocytic line (GL15) (Castellano et al, Arch Pharm 2000; Mariggiò et al, BMC Physiol 2001) has been used to develop an "in vitro" model of astrocyte-neuron coculture. This cell line, together with the human neuroblastoma line SH-SY5Y, has been used for the development of homo- and heterotypic three-dimensional cultures used to verify the effects of microgravity on cell-cell interactions. For this purpose, a rotating cell system on a horizontal axis (Rotating Wall Vessel, RCCS ™ bioreactor) was used to simulate microgravity and, at the same time, allow intercellular interactions. Preliminary data show that stable homo- and heterotypic cell aggregates are formed and remained viable up to one month of culture. Growth under these conditions allows to maintain the phenotypic and functional characteristics of the cells, but determines significant changes in the cytoskeletal structure (Morabito et al, BioMedRes Int 2015). These results are relevant to the use of microgravity as a strategy for the “in vitro” creation of three-dimensional cultures, and show that microgravity is an external stimulus capable of determining changes in shape and, probably, functional activity (Mariggiò and Fanò-Illic Science Proceedings 2015). We developed a quantitative experimental approach to identify modifications of cellular morphology using Normalized Bending Energy and a model of chemoresistant cancer cells (Pasqualato et al, Exp Cell Res 2012). A proposal for a research project was formulated(SHAPE) and approved by the Italian Space Agency. In this project, thanks to the use of a microgravity simulator (random positioning machine, RPM) and cellular models in adhesion and suspension, we are verifying whether and how simulated microgravity conditions interfere with cellular metabolism and redox status. In TCam-2 cells, a model of human male germ cells, the use of antioxidants contrasts the effects induced by simulated microgravity and, moreover, these cells are able to respond with adaptative mechanisms, recovering their normal proliferative activity (Morabito et al, SciReports 2017). In Jurkat cells, expressing the lymphocyte phenotype, microgravity induces an increase in proliferation rate with concomitant reduction of intracellular Ca2+ and ROS. These cells also show adaptive mechanisms (Morabito et al, Int J Mol Sci. 2019).READ MORE
Differentiation mechanisms of multipotent stem cells
Our interest on the study of mechanisms related to cell differentiation includes cells derived from post-mitotic tissues (Fanò et al, Basic Appl Myol 2004). Thanks to the support of a national project, in collaboration with “Sapienza” University in Rome, it was possible to verify in vitro the differentiation potential of satellite cells isolated from skeletal muscle and how this model preserves "memory" of the tissue from which they came from. Satellite cells derived from muscles of transgenic mice overexpressing the mIGF-1 factor show greater sensitivity to trophic stimuli, compared to cells from wild type mice, this accelerates the myogenic process and increases the mobilization of intracellular Ca2+, aspects that seem to emulate muscle hypertrophy observed in the transgenic animal (Guarnieri et al, PlosOne 2014). A collaboration with the University of Ancona led to the functional characterization of a cell model represented by mesenchymal stem cells derived from human skin biopsies, useful as an in vitro model for studying cell differentiation (Orciani et al, Skin Pharmacol and Physiol 2010). In parallel, other models were used to study neurogenesis: amniotic fluid-derived mesenchymal stem cells (AFMSCs) and those derived from the human pulpy nucleus of intervertebral discs. Growing international attention for these models reflects the possibility of using these cells in regenerative medicine as an alternative to those derived from bone marrow. The first phase of the study allowed to confirm the multi-differentiation potential of AFMSCs which, however, “in vitro” are not able to complete their functional neuronal differentiation (Orciani et al, J Biol Regul Homeost Agents 2011). Mesenchymal cells derived from the nucleus pulposus, induced to neuronal differentiation, showed morphological characteristics typical of the neuronal phenotype, but did not develop functional capacities (Lazzarini et al, J Mol Neurosci. 2018). These results encouraged participation in a multidisciplinary study (from basic to clinical research) within the StemTeCh group formed in 2009 by researchers of the Universities "G. d'Annunzio" of Chieti-Pescara and of Teramo. This synergy has also made possible to request and obtain funding. There have been several studies conducted in collaboration, on the model of mesenchymal cells derived from amniotic fluid, including an in-depth characterization of the cells, the definition of the role of calcium-sensing receptors and calcitonin in osteogenic processes and cardiomyocyte differentiation (Di Tomo et al, PlosOne 2013, Morabito et al, Cell Physiol Biochem 2015; Pipino et al, Stem Cells Dev 2015, Di Baldassarre et al, Sci Rep. 2018). Within the StemTeCh group, osteogenic differentiation was also studied in mesenchymal stromal cells from human subcutaneous adipose tissue, investigating the role of purinergic receptors. The results of this study highlighted a pro-osteogenic activity by endogenous ATP and adenosine. Conversely, in presence of high levels of ATP, which occur for example during tissue lesions, osteogenesis is altered (Carluccio et al, Stem Cell Rev. 2019).READ MORE
Role of lymphocytes as biosensors of the integrated functional response
To develop a cell model that could represent a "biosensor” of the adaptive responses of the whole organism we focused on lymphocytes, whose activity, classically related to the immune response, is modulated by physiological and pathophysiological situations. In this context, alterations of the intracellular signalling systems dependent on Ca2+ and on the oxidoreductive status have been revealed in lymphocytes from patients with type 2 diabetes (Belia et al, Free Radic Res 2009). The same intracellular signalling systems are modified, with different magnitude and time-course, in lymphocytes from subjects exposed to high altitude hypobaric hypoxia or from apnea divers after different pre-oxygenation conditions (Mariggiò et al, High Alt Med Biol 2010; Morabito et al, Acta Physiol 2011; Morabito et al, Scand J Med Sci Sports 2016).READ MORE
Determination of functional features using single cell approaches
Based on our expertise and experimental approaches, numerous collaborations have been undertaken, some of which are still ongoing. These include: studies on the mechanisms involved in cell motility (2000-2002 collaboration with Dr M. Falasca, UCL, London UK; Razzini et al, J Biol Chem. 2000; Piccolo et al, Oncogene 2002); the “in vitro” response of various cell types to manganese, which, depending on concentration and permanence in the medium, may behave as a nutrient or as a toxic agent, triggering mechanisms that underlie liver, kidney and nervous disorders (2000-at present collaboration with Prof. G. Mazzoleni, University of Brescia, Brescia Italy; Rovetta et al, Toxicol in Vitro 2007). Scientific collaborations have also been established thanks to the development of single cell methods both in electrophysiology and in fluorescence for the study of the morphology and functional characteristics through the measurement of ionic currents, the analysis of variations in intracellular calcium and/or the production of reactive molecules (NO and ROS). These studies have made it possible to enrich our knowledge of the cellular models used and to underline how the cells, under different conditions (metabolic disorders, stimulation with natural extracts, pharmacological agents or trophic factors) can produce molecules which can act as second messengers or toxic factors (multi-year collaborations still present with different colleagues of G.d’Annunzio University of Chieti-Pescara and Sapienza University of Rome, Di Pietro et al, J Cell Biochem 2006; Bonomini et al, PloSOne 2012; Di Pietro et al, Atherosclerosis 2013; Delli Pizzi et al, PlosOne 2013; Frazzini et al, Cell Death Dis 2016; Dinicola et al, Br J Nutr 2013; Trubiani et al, Cell Signal 2016, Totani et al, BiochimBiophys. Acta 2017, Del Porto et al, PlosOne 2011; Cifani et al, J Cystic Fibrosis 2011; De Rocco et al, Int J Mol Sci 2018). The search for new experimental approaches for the functional characterization of cells and the collaboration with Dr E. Jonas (Department of Internal Medicine, Yale University, USA) allowed to participate in a project aimed to define the role of Bcl-XL in the regulation of neuronal cell metabolism (Alavian et al, Nat Cell Biol 2011; Alavian et al, Brain Res 2012). We also actively participated in a study aimed at standardizing semi-quantitative fluorescence protocols on bone preparations. The data collected so far have revealed that the autofluorescence of bone histological preparations from human remains from different archaeological sites is related to the age of the burial (2010-2018, collaboration with Prof. L. Capasso, University G.d’Annunzio, Chieti Italy; Capasso et al, Forensic Sci Int 2017). The use of fluorescence videoimaging techniques, complemented by functional experimental approaches, also allowed to study the interactions of our cell models with biocompatible materials to develop applications in the field of biomedicine (collaborations with different colleagues of Gd’Annunzio University of Chieti-Pescara, Sinjiari et al, J Biol Regul Homeost Agents 2012; Lanuti et al, Biores Open Access 2015; Cataldi et al, J Mater Sci Mater Med 2016).READ MORE



