Development of Antibody-Drug Conjugates for Cancer Treatment (Group Leader: Prof. G. Sala)
Antibody–drug conjugates (ADCs) are pharmaceutical compounds created to meet the need to combine high tumor-killing activity with the lowest off-target toxicity. They represent one of the most successful examples of targeted therapy in oncology. Indeed, cancer treatment has seen significant advances and growth in terms of therapy options, thanks to a significant increase in carriers available for selective drug delivery.
ADCs have complex molecular structures, including a highly selective monoclonal antibody (mAb) directed against a tumor antigen, potent cytotoxic small molecule (payload), and linker connecting these two moieties. The chemistry used to link the toxin to the antibody backbone and DAR (drug–antibody ratio) may play a critical role in the activity of an ADC, both influencing cell killing activity and off-target toxicity as well as pharmacokinetic properties. Two main categories of highly potent cytotoxic anticancer agents are used in antibody–drug conjugates: microtubule-disrupting agents and DNA-modifying agents. The choice of the payload used to arm the antibody is a prime step in the development of an ADC as the sensitivity to cytotoxic agents varies between tumors of different origin. Finally, ADCs can be directed against both tumor and stromal components, constructed using internalizing or non-internalizing antibodies, and different mechanisms of action can be designed to increase the therapeutic activity of an ADC according to the used target.
HER-3 Project
HER-3 receptor plays a crucial role in modulating the sensitivity of targeted therapeutics in different cancers. Indeed, upregulation of HER-3 expression and activity is being considered as one of the most common mechanism used by tumor cells as escape mechanisms. Therefore, targeting this receptor may represent an efficient strategy to enhance therapy efficacy in several types of cancer. We have developed and ADC against HER-3 named EV20/MMAF which showed potent therapeutic activity in melanoma and breast cancer tumors resistant to anti-HER-2 therapeutics.
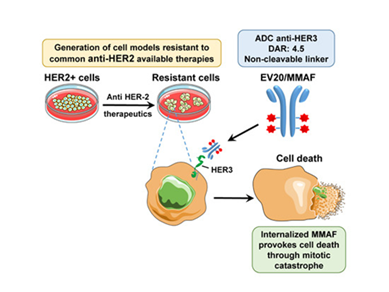
This study shows the therapeutic value of EV20/MMAF, a HER3‐targeted antibody‐drug conjugate loaded with monomethyl auristatin F, for the treatment of breast tumors refractory to conventional anti‐HER2 therapies.
- HER3 was expressed in HER2 positive breast cancer cells with (acquired) resistance to anti‐HER2 therapies.
- The antibody drug conjugate EV20/MMAF, which targets HER3, exerted a strong antitumoral action in several models of primary and secondary resistance to commonly used anti‐HER2 therapies.
In mice bearing tumors resistant to trastuzumab or trastuzumab‐emtansine, single‐dose EV20/MMAF caused long‐lasting tumor regression without relapse.
LGALS3BP Project
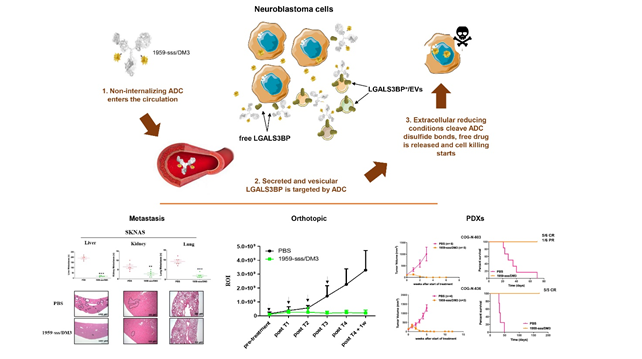
LGALS3BP (Galectin- 3 binding protein, aka Mac-2 BP or 90K), is a human secreted protein expressed by the large majority of human cancers, while being virtually undetectable or expressed at low levels in normal human tissues. Whereas the bio-physiological role of LGALS3BP is not yet fully understood, accumulating evidence has shown that the protein may be involved in cancer growth and progression. Notably, significantly elevated expression of LGALS3BP in the serum or tumor tissue has been found to be associated with poor clinical outcome in patients with a variety of malignancies, including neuroblastoma and glioblastoma. Recently, Gal-3BP is found to be one of the most abundant surface component of cancer-derived Extracellular Vesicles (EV)s . EVs have a prominent role in inducing a TME that is favorable to cancer progression through the induction of immune escape, the stimulation of angiogenesis and preparation of the pre-metastatic niche by educating BM-derived progenitor cells. Our aim is to validate LGALS3BP as a suitable target for ADC therapy and provides a rationale for further clinical development of an anti-LGALS3BP based ADC for the treatment of neuroblastomas and other Gal-3BP+ tumors.