The antiplatelet agent Revacept prevents the increase of systemic thromboxane A2
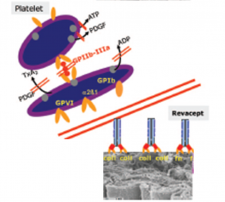
Paola Patrignani’s group has recently published in Scientific Reports (manuscript accepted on November 13, 2020) that the novel antiplatelet agent Revacept can mitigate vascular neointima hyperplasia in a femoral artery injury mouse model. Revacept is a biotechnological drug obtained by the fusing the extracellular domain of GPVI (the main platelet collagen receptor) with the human immunoglobulin (IgG) Fc domain; it can bind specifically to collagen at vascular damage sites. Revacept can represent a promising therapeutic strategy to prevent restenosis in patients with coronary artery disease treated with percutaneous transluminal coronary angioplasty and stent implantation. This will be verified in a phase II randomized, double blind trial which is ongoing in patients undergoing elective percutaneous coronary intervention (ISAR24 PLASTER Trial).
This study is an international collaboration with Prof. Ying Yu (Shanghai Institute for Biological Sciences, Chinese Academy of Science, Shanghai, China) and Dr. Gotz Münch (AdvanceCOR GmbH, Martinsried, Germany).
Title: The antiplatelet agent Revacept prevents the increase of systemic thromboxane A2 biosynthesis and neointima hyperplasia
Authors: Sara Alberti,* Qianqian Zhang,* Ilaria D’Agostino,* Annalisa Bruno, Stefania Tacconelli, Annalisa Contursi, Simone Guarnieri, Melania Dovizio, Lorenza Falcone, Patrizia Ballerini, Gotz Münch, Ying Yu, and Paola Patrignani
*These authors contributed equally
Revacept’s mechanism of action. Ungerer et al. Circulation. 2011; 123: 1891-1899