Physiology and Physiopathology of Striated Muscles
Group Leader: Feliciano PROTASI – Full Professor of Physiology
Description of Protasi Group
The main focus of the Protasi laboratory is to characterize the mechanisms that control intracellular calcium levels in striated muscles in health (basic-science) and disease (applied-science).
Muscle fibers/cells contracts, relax, produce energy, and adapts to exercise, disuse and ageing thanks to changes in intracellular calcium levels that are finely modulated. Calcium maybe either released from intracellular stores or enter form the extracellular space thanks to two mechanisms:
- Excitation-contraction (EC) coupling
READ MORE
In skeletal muscle fibers, an extremely well organized system of tubules and vesicles, collectively named sarcotubular system, is able to finely control the cytoplasmic calcium (Ca2+) concentration. The sarcotubular system is formed by two separate systems of membranes: transverse tubules (TTs) and sarcoplasmic reticulum (SR). The communication between these two membranes in triads (also named Ca2+ release units) allows the transduction of the electrical signal carried by TTs into a release of Ca2+ from the SR, a mechanism called excitation-contraction (EC) coupling. Dihydropyridine receptor (DHPR), ryanodine receptor type-1 (RyR1), and calsequestrin-1 (CASQ1) are the three main proteins involved in this mechanism. The DHPR (or Cav 1.1) is an L‑type Ca2+ channel of exterior membranes responsible for initiating EC coupling by triggering Ca2+ release from RyR1, a channel located in the SR terminal cisternae. CASQ1, a Ca2+ binding protein localized in the SR lumen, increases the capability of the SR to store the Ca2+ needed to activate muscle contraction.
Excitation-contraction (EC) coupling
- Store-operated calcium entry (SOCE)
READ MORE
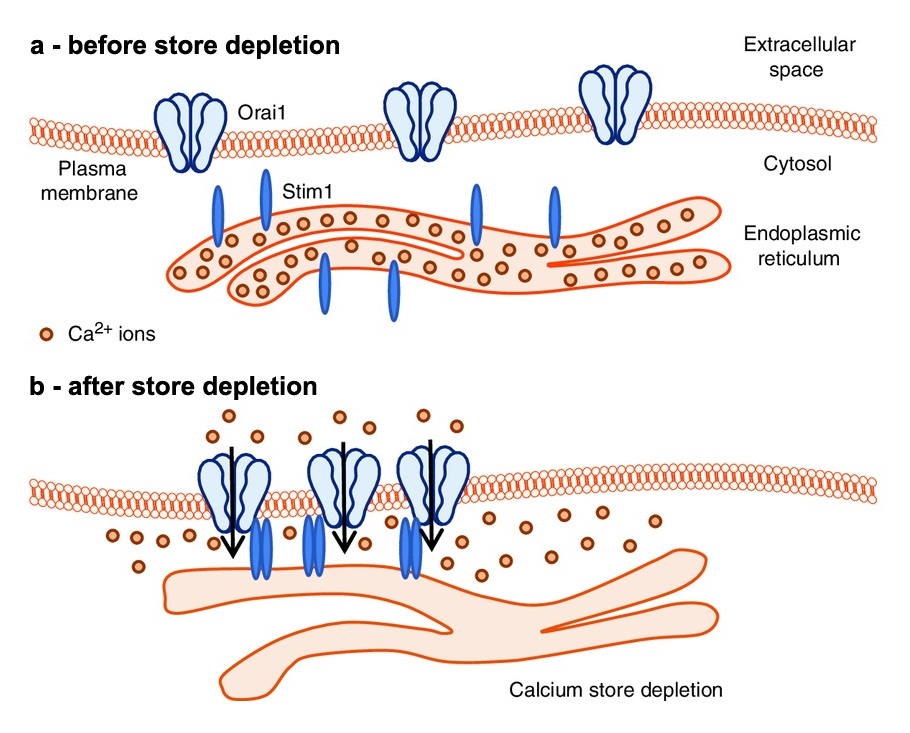
Store-operated Ca2+ entry (SOCE) is a Ca2+ entry mechanism, first described in non-excitable cells, that is triggered by depletion of intracellular Ca2+ stores (endoplamic and sarcoplasmic reticulum, ER and SR).
SOCE is coordinated by the interaction between: a) STIM1, which acts as the Ca2+ sensor in the ER/SR; and b) Orai1, a Ca2+-permeable channel of the plasma membrane (PM). The mechanism that lead to activation of SOCE involves i) Ca2+ depletion of internal stores, ii) conformational changes that induces dimerization of STIM1 proteins, and finally iii) translocation towards the PM of STIM1-dimers that acivates Orai1.
Being first reported in 2001, SOCE is a relatively new phenomenon in skeletal muscle, where it is also mediated by interactions between STIM1 in the SR and Orai1 in transverse tubules (TTs), specialized invaginations of the PM that carry the action potential. Recently, also Calsequestrin-1 (CASQ1), a protein that acts as the main Ca2+ buffer in the SR, has been proposed to modulate SOCE by a retrograde signal that inhibits STIM1 aggregation. In muscle, SOCE is required for development of contractile function, and to limit muscle fatigue during repetitive stimulation.
Two main projects running in the lab are mainly of basic-science nature (1 and 2), while applied-science projects are focused in unraveling the patho-physiological mechanisms underlying myopathies caused by mutation/dysfunction of proteins involved in EC coupling (3) and SOCE (4):
- Identify and characterize the sites of Store-operated Ca2+ Entry
READ MORE
Activation of store-operated Ca2+ Entry (SOCE) requires an interaction between STIM1, the Ca2+ sensor of the sarcoplasmic reticulum (SR), and Orai1, a Ca2+ release activated channel (CRAC) placed in transverse tubules (TTs). It has been suggested that the rapid kinetics of SOCE activation in skeletal muscle would require a preformed site of interaction between STIM1 and Orai1. As in pre-formed triad junctions (also named Ca2+ release units) TTs and SR are already associated to each other to mediate excitation-contraction (EC) coupling, this site has been proposed for years as the perfect location for SOCE. Nevertheless, it should acknowledged that some peculiar features of triad junctions may represent a limit for proper STIM1-Orai1 interaction:
a. The junctional gap at the triads is a quite crowded space, where many proteins such as RyR, DHPRs, junctiphilins, FKBP12, triadin, junctin, mitzugumin, STAC3, etc. modulate EC coupling.
b. The junctional gap in triads is 12nm wide, whereas STIM1 overexpressed in non-muscle cells forms stacks of ER in which the space between membranes isonly about 8 nm.
Hence, the possibility existed that SOCE may occur at sites different from the triads. Recently published evidence from our laboratory suggested that the most likely site for SOCE may indeed not be the triad (Boncompagni et al. 2017; Michelucci et al. 2019). This project is currently funded by: a) NIH-NIAMS Funds RO1 AR059646-06; and b) Italian Telethon GGP19231.
(Figure 1)
- Investigate the communication between Ca2+ release units and mitochondria
READ MORE
Calcium ions (Ca2+) are versatile second messengers that play a key role in a variety of cellular physiological functions, including gene expression, differentiation, neurotransmitter release, muscle contraction, motility, and communication between cells.Bi-directional Ca2+ signaling between mitochondria and intracellular stores (endoplamic and sarcoplasmic reticulum, ER and SR) underlies important cellular functions, including oxidative ATP production. Intimate structural interactions between ER and mitochondria have been demonstrated in some cell types. In skeletal muscle, available morphological data suggested a close proximity of a large fraction of mitochondria to the SR, in proximity of sites of Ca2+ release (i.e. triads).
I the last 10 years we have been studying the interaction between triads and mitochondria in different skeletal muscle models (development, ageing, exercise, denervation, and rare diseases) to gather information about the importance of a proper triad-mitochondria association for efficient muscle function (Boncompagni et al. 2009; Zampieri et al. 2015; Pietrangelo et al. 2019).
(Figure 2)
- Link between malignant hyperthermia susceptibility (MHS) and exertional-environmental heat stroke (EHS)
READ MORE
Malignant hyperthermia susceptibility (MHS1) is a subclinical myopathy characterized by a hypermetabolic response to volatile anaesthetics (such as halothane), which can be life-threatening if not corrected immediately by suspension of triggering agent and treatment with Dantrolene. The disorder is generally associated to gain-of-function mutations of RyR1, the Ca2+ release channel of the sarcoplasmic reticulum (SR). Interestingly, life-threatening over-heating episodes have been reported in individuals also after exposure to environmental heat, physical exertion, or even during febrile illness. EHS has recently become reason of health concern, because of an increased frequency and severity of unusual heat-waves coupled with the absence of any pharmacological interventions to prevent/reverse EHS episodes. Animal studies support the hypothesis of MHS1 and EHS being related disorders: pigs carrying mutations in RyR1 (Porcine Stress Syndrome) and knock-in mice carrying specific human RyR1 mutations linked to MHS1 undergo MH episodes in response to halothane, but also to heat. We have recently discovered that also mice lacking calsequestrin-1 (CASQ1), a proteins that accumulates Ca2+ in the SR and that modulate the activity of RyR1, trigger MHS episodes not only during anaesthesia, but also if exposed to heat and even exercise (Dainese et al. 2009; Protasi et al. 2009; Michelucci et al. 2017). This project was funded by: a) Italian Telethon ONLUS GGP08153 and GGP13213; and b) NIH-NIAMS Funds RO1 AR053349-06. Funding for this project is being seeked.
(Figure 3)
- Role of STIM1 and Orai1 in the formation of tubular aggregates (TA) in ageing and TA myopathy (TAM)
READ MORE
Dysregulation of Ca2+ homeostasis is associated to several pathological conditions including skeletal muscle diseases. STIM1, which acts as the Ca2+ sensor in the endoplamic and sarcoplasmic reticulum (ER and SR), and Orai1, a Ca2+-permeable channel of the plasma membrane (PM), are the two main actors in Store-operated Ca2+ Entry (SOCE), a mechanism that contributes to Ca2+ homeostasis. Tubular aggregates (TAs) are unusual assemblies of SR membranes, preferentially found in ageing fast twitch fibers. Gain-of-function mutations in STIM1 and Orai1 genes, encoding for the two proteins which mediate SOCE, are responsible of a rare form of myopathy known as TA myopathy (TAM). Recently, also three novel mutations in the calsequestrin-1 (CASQ1) gene, encoding for a protein proposed to regulate SOCE, have been linked to TAM patients. In this project, we are studying a) TAs in ageing muscle of mice and b) mechanisms linking human mutations to dysfunction of SOCE in newly generated knockin mice. This project is currently funded by Italian Telethon GGP19231.
(Figure 4)
The laboratory approaches these projects integrating the use of transgenic/knock-in/knock-out mouse models and human biopsies with structural and functional techniques ranging from electron microscopy (see EM Facility), confocal imaging, to biochemistry, in vitro measurements of muscle contractile properties, behavioral measures of in vivo muscle performance (treadmill, grip strength), indirect calorimetry, etc.
The Research Unit of Prof. Protasi occupies the following spaces within CAST:
1. The Electron Microscopy (EM) Laboratory (see EM Facility)

2. The Biochemistry Laboratory (~180 square feet) is located on the second floor of CAST and is equipped with a chemical hood and has a lab-office carrel with computer to be used by personnel working in the room.

4. The Real-Time PCR Laboratory (~180 square feet), is located on the third floor of CeSI-Met, and is equipped with a chemical hood and RNAase free lab-space, a spectrophotometer connected to a comupter positioned on a lab-office carrel to be used by personnel working in the room.

5. Laboratory of Physiology in-vivo vivo (215 square feet) is located in the basement of the building, in proximity of the Animal Facility and is completely dedicated to in-vivo testing of animal models (treadmill, environmental chamber, grip test, indirect-calorimetry system, etc.).

Office Space and Workstations:
3 offices are available for the PI (F. Protasi) and Assistant Professor (C. Paolini).
In addition:
- an office equipped with two work stations is shared by two members of the EM Facility :
6 additional lab-office carrels (each one equipped with computers) are available for lab members in the different lab spaces.
Electron Microscope (see EM facility)
Ultra-microtomes (see EM facility)
Aurora Scientific System for functional studies in muscles:
Aurora 1300A: 3-in-1 muscle test system for functional evaluation of intact muscles in-situ (300C apparatus) and ex-vivo (800A apparatus; Aurora Scientific Inc., Ireland).
The 1300A is high performing, precise test systems to test tensile and other mechanical properties of muscles. The test systems has a mouse apparatus, complete with temperature-controlled animal and limb plates designed to support and fix the animal and limb being tested. The system can be converted to an isolated (in-vitro) muscle test system with the attachment of a 25mL bath, to measure force and length in muscle samples. A control and analysis software (DMC/DMA) is used to test parameters such as resting length, resting force, and stimulation.

Indirect Calorimetry System (Panlab, Spain):
OxyletPro is equipped with two physiocages and a one-lane treadmill & Metabolism Software (Panlab, Spain)
- OxyletPro-physiocage is a modular system for studies in laboratory research models, utilizes indirect calorimetry to evaluate respiratory metabolism (VO2 consumption/VCO2 production) in mice.
- OxyletPro-Treadmill apparatus consists of a rolling belt with adjustable speed (up to 150 cm/s) and slope (from -25 to 25 degrees) and a control Unit. The treadmill unit controls the speed of the belt, shows measured data in its touchscreen display and provides electrical shock to the grid.
For metabolism studies, the treadmill is provided with an air isolated enclosure. The associated METABOLISM software transfers the data from the treadmill control unit and the gas analyzer to a PC computer using RS232/USB outputs for data storage and further analysis.
The Metabolism software offers modules for respiratory metabolism (METAOXY) allowing storage and further analysis of data from physiocages and treadmill.

Treadmill for mice (Columbus Instruments, OH):
Exer-6M is a general purpose six lane animal exerciser utilizing single belt construction with dividing walls suspended over the tread surface. Exer-6 is supplied with a stimulus assembly that is composed six shock grids, each with individual on/off switches. Stimulus intensity is adjustable and LED lamps indicate which stimulus grid is active. Design of electrical stimulus grid reflects special attention to avoid injuries to animals. An included drive motor controller provides smooth and continuous adjustment of speed in the range 3-100 m/m. Inclination option is also available. The Exer-6M has a unique wide range of speed adjustable from 3 to 100 meters per minute and adjustable acceleration in 0.1 meter/minute-steps per second.

Mouse Running Wheels for voluntary activity (Columbus Instruments, OH).
16 Station home cage Running Wheel System with Multi Device Interface software (Columbus Instruments, OH). The system measures spontaneous activity in a voluntary free-spinning running wheel. Each wheel has a magnetic indicator and a hall effect sensor that connects to a computer interface and records wheel revolutions at user-specified intervals. Data can be viewed in real time and exported at the end of the experiment with the DI software. With the knowledge of interval duration, number of revolutions, and wheel circumference, calculations for time, distance, and speed in each bout are all possible.

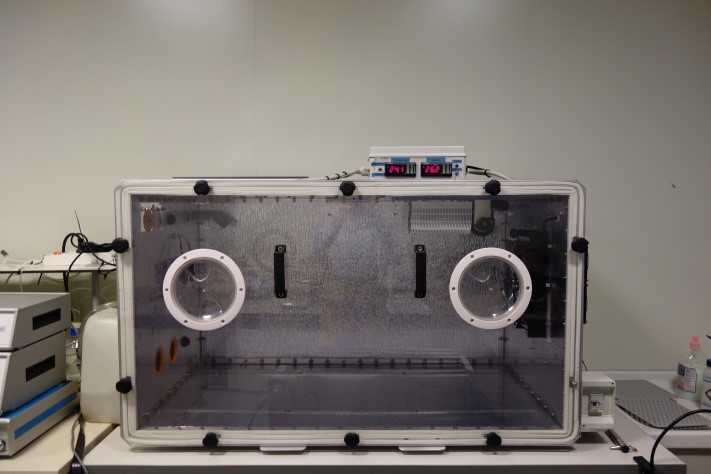
Environmental chamber (custom made)
The environmental chamber is a climatic chamber that can manage temperature and humidity in wider and more varied conditions than standard ones. The chamber is equipped with a window that allows an internal view during the experiments. Climatic conditions are finely regulated by sensors (humidity from 0 to 100% and temperature from 20 up to 45°C). The chamber has an internal space of that can fit treadmill and chambers for measurements of oxygen consumption.
77 - Paolini, C., A. Michelucci, M. Quarta, L. Wei-Lapierre, A. Nori, C. Reggiani, R.T. Dirksen and F. Protasi. 2015. Oxidative stress, mitochondrial damage, and cores in muscle from calsequestrin-1 knockout mice. Skeletal Muscle. 5:10. doi: 10.1186/s13395-015-0035-9
78 - Michelucci, A., C. Paolini, M. Canato, L. Wei-Lapierre, L. Pietrangelo, A. De Marco, C. Reggiani, R.T. Dirksen, and F. Protasi. 2015. Anti-oxidants protects calsequestrin-1 knockout mice from halothane- and heat- induced sudden death. Anesthesiology. 123:603-617. doi: 10.1097/ALN.0000000000000748
80 - Pietrangelo, L., A. D'Incecco, A. Ainbinder, A. Michelucci, H. Kern, R.T. Dirksen, S. Boncompagni, and F. Protasi. 2015. Age-dependent uncoupling of mitochondria from Ca2+ release units in skeletal muscle. Oncotarget. 6: 35358-35371. doi: 10.18632/oncotarget.6139
87 - Michelucci, A., C. Paolini, S. Boncompagni, M. Canato, S. C. Reggiani, and F. Protasi. 2017. Strenuous exercise triggers a life-threatening response in mice susceptible to Malignant Hyperthermia. Faseb J. 31:3649-3662. doi: 10.1096/fj.201601292R
90 - Michelucci, A., S. Boncompagni, M. Canato, C. Reggiani, and F. Protasi. 2017. Estrogens protect Calsequestrin-1 knockout mice from lethal hyperthermic episodes by reducing oxidative stress in muscle. Oxidative Medicine and Cellular Longevity. 2017:6936897. doi: 10.1155/2017/6936897
91 - Boncompagni, S., A. Michelucci, L. Pietrangelo, R. T. Dirksen, and F. Protasi. 2017. Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Scientific Reports. 7(1):14286. doi: 10.1038/s41598-017-14134-0
92 - Guarnier, F.A., A. Michelucci, M. Serano, L. Pietrangelo, C. Pecorai, S. Boncompagni, and F. Protasi. 2018. Aerobic training prevents heatstrokes in calsequestrin-1 knockout mice by reducing oxidative stress Oxidative Medicine and Cellular Longevity. 2018:4652480. doi: 10.1155/2018/4652480
101 - Iodice, P., S. Boncompagni, L. Pietrangelo, L. Galli, E. Pierantozzi, D. Rossi, A. Fusella, M. Caulo, H. Kern, V. Sorrentino, and F. Protasi. 2019. Functional Electrical Stimulation: a possible strategy to improve muscle function in Central Core Disease? Front. Neurol. 10:479. doi: 10.3389/fneur.2019.00479
103 - Michelucci, A., S. Boncompagni, L. Pietrangelo, M. García-Castañeda, T. Takano, S. Malik, R.T. Dirksen, and F. Protasi. 2019. Transverse tubule remodeling enhances Orai1-dependent Ca2+ entry in skeletal muscle. eLife. 8.e47576. doi: 10.7554/eLife.47576
 Laura PietrangeloJunior Researcher (Ric. TDA) |
||





