Laboratory Of Diabetes, Metabolism And Endocrinology
This group’s main focus is the study of the cellular mechanisms underlying the vascular complications of diabetes mellitus as well as research on the impact of innovative treatments for type 2 diabetes on glucose control, cardiovascular risk factors and endothelial dysfunction.
This is pursued by in vivo, ex-vivo and in vitro studies.
As to the in vivo studies, conducted at the Clinical Research Center, this group has participated in the last few years in over 25 multicenter clinical studies testing the efficacy and safety of innovative diabetes treatments. Among these, several were trials aimed at exploring cardiovascular outcomes in subjects treated with GLP-1 receptor agonists, SGLT2 inhibitors or innovative long-acting insulin analogues. In particular, Professor Consoli has been the National Coordinator for the DEVOTE (insulin degludec), the SUSTAIN 6 (semaglutide), The PIONEER 6 and the SOUL (oral semaglutide) studies.
Several investigator-initiated in vivo studies have been conducted and are presently in progress at the Clinical Research Center, aimed at exploring peculiar effects of GLP-1 receptor agonists and SGLT2 inhibitors on body composition, beta cell function, NAFLD, innovative biomarkers of endothelial health and cognitive function.
From Baldassarre MPA et al., Diabetes Obes Metab. 2018
As to the ex-vivo and in vitro studies, they are mainly focused on endothelial dysfunction.
What is generally referred to as endothelial dysfunction should more appropriately be considered as endothelial activation, which may eventually contribute to arterial disease when certain conditions are fulfilled. Endothelial activation represents a switch from a quiescent phenotype toward one that involves the host defence response. Indeed, most cardiovascular risk factors activate a molecular machinery in the endothelium resulting in increased oxidative stress, expression of mediators of inflammation and adhesion molecules designed to interact with leukocytes and platelets and target inflammation to specific tissues to clear microorganisms.
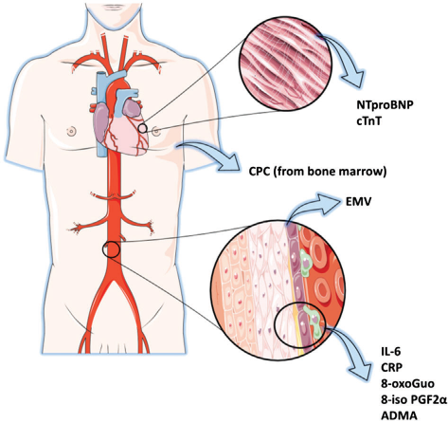
One of the main interests of our lab is to phenotype human umbilical vein endothelial cells (HUVECs) chronically exposed to hyperglycaemia and to a pro-inflammatory environment during pregnancy, so as to identify molecular modifications of cellular homeostasis eventually impacting on endothelial dysfunction. Such cells are harvested from umbilical cord vessels of women affected by gestational diabetes, a condition associated with increased oxidativestress and overexpression of inflammatory cytokines, both of which might lead to endothelial dysfunction and vascular disease. Besides characterizing these cells for molecular end epigenetic aspects differentiating them from cells obtained by control women, we use them to study the effects of various compounds (both diabetes drugs and nutraceutical substances), in order to evaluate their ability to modulate pro-atherosclerotic alterations. Umbilical cords are mostly harvested from women followed at our gestational diabetes clinic attended by about 700 pregnant diabetic women per year. This allows comparisons between cellular characteristics and clinical features of the donors. Endothelial dysfunction is an early event during the pathophysiologic process of vascular damage related to atherosclerotic plaque formation. It provides an important link between diabetes and the high risk for cardiovascular events that diabetic patients exhibit. Modificato da Lawson et al, Microvesicles and exosomes: new players in metabolic and cardiovascular disease Journal of Endocrinology (2016) 228, R57–R71 READ MORE
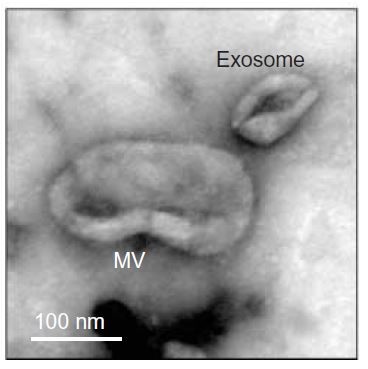 Emerging evidence suggests that extracellular vesicles (EVs) could serve as specific biomarkers of endothelial health. These biomarkers may be a useful tool in order to detect and follow vascular status in diabetic patients in response to therapies. This is important considering the currently available evidence regarding the new glucose-reducing therapies with proven cardiovascular benefit and the need of deepen the protective effect of these new agents on vascular wall and on cardiovascular risk reduction. A reliable and rapid method for the assessment of vascular status could be an important tool in order to identify and quantify diabetes induced vascular damage. Our group is now testing, in vivo, the extracellular vesicles deriving from endothelium, platelets and leucocytes (thus from cells directly involved in the atherosclerotic vascular alterations) as potential biomarker of vascular modification. We are also investigating the potential role of this marker in monitoring vascular benefit of different agents for type 2 diabetes treatment.
Emerging evidence suggests that extracellular vesicles (EVs) could serve as specific biomarkers of endothelial health. These biomarkers may be a useful tool in order to detect and follow vascular status in diabetic patients in response to therapies. This is important considering the currently available evidence regarding the new glucose-reducing therapies with proven cardiovascular benefit and the need of deepen the protective effect of these new agents on vascular wall and on cardiovascular risk reduction. A reliable and rapid method for the assessment of vascular status could be an important tool in order to identify and quantify diabetes induced vascular damage. Our group is now testing, in vivo, the extracellular vesicles deriving from endothelium, platelets and leucocytes (thus from cells directly involved in the atherosclerotic vascular alterations) as potential biomarker of vascular modification. We are also investigating the potential role of this marker in monitoring vascular benefit of different agents for type 2 diabetes treatment.
A further line of investigation our group is pursuing is related to the impact that consumption of a Low Carb diet might have on metabolic parameters.
Diet is a key factor for improving metabolic parameters and favour weight control in diabetic patients. The Mediterranean diet, mostly composed of whole grain and low glycemic index foods, assumed in whole meals rich in complex carbohydrates, proteins of high biological value, and mono and polyunsaturated fats, has been proven efficacious in reducing metabolic and CV risk. Substitution and/or supplementation of table food with specific low glycemic index/high fibres products might help in gaining the benefit of a Mediterranean diet. However, products with a low glycemic index currently on the market present several problems, such as unbalanced ratio of carbohydrates and proteins, allergenic flours, high cost organoleptic properties not always well accepted by the consumers.Our group is testing a range of food products with high fibres content and low glycemic index (very similar to the common food in appearance and taste) to be used as substitute foods in association with a balanced Mediterranean diet, exploring their effects in term ofweight control and improvement of blood glucose profiles both in obese and diabetic patients.READ MORE
Modified from da Giuliani C et al., PLoS ONE 9(9): e107936.a
Finally, our research interests include the study of autoimmune thyroid disorders, specifically:
- Regulation of major histocompatibility complex (MHC) class I and class II genes expression in thyroid cells and their relationship with the development of autoimmune thyroid diseases
Abnormal expression of MHC Class I and II is associated with thyroid autoimmunity. Aberrant class II, together with abnormal TSH receptor (TSHR) expression, can induce autoimmune hyperthyroidism and a Graves’-like syndrome in mice. Abnormal class I expression is also linked to Graves’ disease, since class I suppression has been shown to be an important component of the immunosuppressive action of methimazole. Evidence has accumulated that hormones and growth factors that regulate thyroid cell growth and function can coordinately decrease MHC class I expression and may prevent autoimmune thyroid disease. Our studies are performed in vitro on a reliable model of thyroid cells in continous culture, the FRTL-5 cells. They evaluate the effects of hormones, growth factors, cytokines and drugs on MHC class I and II transcription troughout the inquiry of the binding of the transcription factors to their promoters.READ MORE
- NOD.H-2h4 mice and thyroiditis
Autoimmune thyroiditis (AT), also known as Hashimoto’s thyroiditis is a common autoimmune disease. Its etiology is multifactorial with genetic and environmental factors contributions, such as iodine. Excess dietary Iodine can exacerbate thyroiditis in genetically susceptible hosts, such as the NOD.H-2h4 mice. The mechanisms by which iodine triggers thyroid autoimmunity remains not fully understood. The overexpression of reactive oxygen species (ROS) has been proposed as one of them. We are using the NOD.H-2h4 mouse model of spontaneous thyroiditis for two aims: 1) to clarify the role of reactive oxygen species in AT pathogenesis and 2) to evaluate the role of minor tobacco alkaloids in reducing ROS ant thus ameliorating lymphocytic infiltration and production of thyroid autoantibodies, the hallmarks of AT.READ MORE
- TSH receptor autoantibodies (TRAbs)
Since the initial evidence for the presence of a serum thyroid-stimulating factor in Graves’ disease (GD) studies have shown that TRAbs are functionally heterogeneous; some stimulating TSHr (TSAb), with others with blocking (TSBAb) activity. Notably, TSAb and TSBAb may coexist in the same patient, varying in amount with time and making the clinical picture complex and difficult. The clinical utility of the TRAb routine assay, which is usually performed in most clinical labs, is therefore limited since it measures the sum of TSAb and TSBAb. Our original goal was to create a bioassay that might measure only TSAb functional activity and have a clear cutoff between Graves’ patients and controls. In the following step a different bioassay which measure only TSBAb activity has been created. Accordingly, bioassays have been created by using cells stably transfected with different chimeras which retain either the agonist sensitive TSAb epitope on the N-terminal portion of the extracellular domain of the human TSHr or the TSBAb epitope on the C-terminal portion. Our studies are performed using mammalian cells expressing TSH receptor-LH/CG receptor chimeras that activate a luciferase reporter gene. The bioassay allows to evaluate the distinct functional TRAbs (stimulating, blocking, neutral) and to correlate their presence with the clinical and prognostic features of thyroid autoimmune diseases (both Graves’ disease and Hashimoto’s thyroiditis).READ MORE
- Polyphenols as endocrine disruptors
We are performing in vitro (FRTL-5 cells) and in vivo animal (Sprague-Dawley rats) studies to evaluate the effects of quercetin and resveratrol on thyroid growth and function.READ MORE







