KDEL receptor a novel regulator of cancer cell growth and progression (Group Leader: Prof. Michele Sallese)
The KDEL receptor (KDELR) was discovered in the 80s of the last century. Its primary function was to bring back to the endoplasmic reticulum (ER) the chaperones that leaked out during the secretory process. Subsequently, it became clear that KDELR participates in ER quality control and the unfolded protein response (UPR).
Our research group discovered completely novel regulatory circuitries based on ER and Golgi endomembranes. We showed that the KDELR functions as a G-protein coupled receptor (GPCR) and couples to and activates the heterotrimeric G-proteins Gαq/11 and Gαs, when it is bounded by chaperones. This signalling cascade finally leads to the activation of PKA and Src oncogene (Fig. 1).
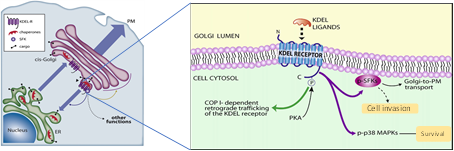
Chaperones containing KDEL sequences leave the ER with cargo. On arrival at the Golgi, they bind to the KDELR triggering the activation of PKA, SFKs and p38 MAPKs. In turn, these kinases are necessary for the regulation of retrograde and anterograde traffic machinery. Remarkably, KDELR-dependent kinase activation can also regulate other key functions in the cell including cell invasion and survival.
The KDELR-PKA signalling phosphorylates a number of proteins involved in membrane trafficking although, remarkably, many other proteins play a role in cell growth, motility, energy metabolism and transcription. Prompted by the central role of Src in invadopodia formation and cell invasion, we showed that activation of KDELR-Src pathway promotes the formation/activation of invadopodia and extracellular matrix degradation in A375 melanoma cells, whereas its inhibition resulted in an impairment of cell degrading performance (Fig. 2).
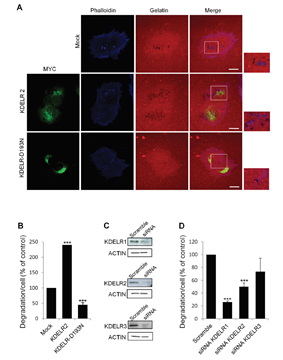
More recently, we discovered that KDELR signalling cascade targets focal adhesion kinase (FAK) on focal adhesions and invadopodia. These findings pose the KDELR as a relevant target to oppose metastasis formation.
Moreover, the research interest of M. Sallese also covers fundamental research and other diseases:
Pathological mechanisms involved in Marinesco-Sjogren syndrome and possible therapeutic strategies
MSS is a rare, at present incurable, autosomal recessive disorder of infancy characterized by muscle weakness and ataxia. About 60% of MSS patients carry homozygous or compound heterozygous SIL1 mutations that make the SIL1 protein unstable, eventually leading to its loss. The SIL1 protein is an ATP-exchange factor for BiP, the master chaperone of the endoplasmic reticulum (ER). In the case of SIL1 loss, BiP remains associated with its client protein, leading to accumulation of unfolded proteins, ER stress and activation of the unfolded protein response (UPR) (Fig. 1), which in turn causes cerebellar Purkinje cell death and myopathy.
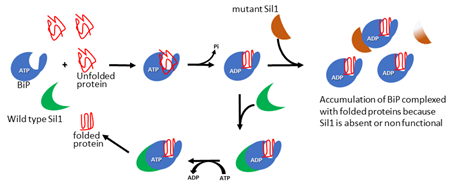
Basics of Marinesco-Sjogren syndrome. Immunoglobulin‐binding protein (BiP)‐dependent protein folding requires suppressor of Ire1/Lhs1 double mutant (SIL1). The interaction of unfolded proteins with ATP‐bound BiP triggers the hydrolysis of ATP and a conformational change in BiP that promotes folding of the client protein. The folded substrate is released from BiP thanks to SIL1 that catalyses the exchange of ADP with a new molecule of ATP. The folded protein is released and ATP‐bound BiP is ready to start a new folding cycle. When SIL1 is non‐functional or absent, BiP remains associated with the folded protein, which cannot be delivered to its final destination. BiP is not available for further folding cycles and newly synthesised unfolded proteins accumulate in the endoplasmic reticulum.
We used HeLa cells to test the hypothesis that impaired protein folding in the ER due to loss of SIL1 could affect secretory pathway, impairing the transport of cargoes essential for the function of MSS vulnerable cells. Immunofluorescence analysis of SIL1-knockdown (KD) cells detected ER chaperone aggregation, enlargement of the Golgi complex and increased autophagic vacuoles (Fig. 2).
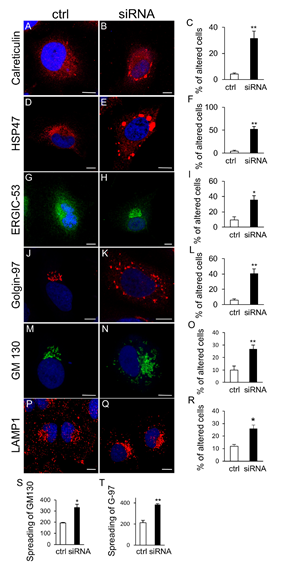
SIL1-KD cells also had delayed ER to-plasma membrane transport with retention of Na(+)/K(+)-ATPase and procollagen-I in the ER and Golgi, and increased apoptotic rate. The PERK pathway of the unfolded protein response was activated in SIL1-KD cells, and the PERK inhibitor GSK2606414 attenuated the morphological and functional alterations of the secretory pathway, and significantly reduced cell death. These results indicated that loss of SIL1 was associated with alterations of secretory transport, and suggested that inhibiting PERK signalling might alleviate the cellular pathology of SIL1-related MSS.
Pre-symptomatic treatment of woozy mice with GSK2606414 delayed neurodegeneration and the onset of motor deficits, prolonging the asymptomatic phase of the disease, and improved muscle pathology and motor performance in the symptomatic phase (Fig. 3).
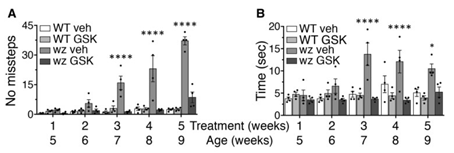
However, GSK2606414 is toxic to the pancreas and does not completely rescue the woozy phenotype, possibly because full translational recovery due to complete PERK inhibition overloads the inefficient folding machinery of SIL1-deficient cells. It may be necessary to fine-tune PERK signalling for better effects.
Our finding indicate that targeting PERK signalling may have beneficial disease-modifying effects in MSS, but this therapeutic approach needs to be optimized for better, long-lasting results.
Identification of biomarkers and pathological mechanisms of non-celiac wheat sensitivity (NCWS)
Non-celiac gluten/wheat sensitivity (NCGS/NCWS) is a syndrome dependent on gluten/wheat ingestion that presents both intestinal and extra-intestinal symptoms.
NCWS clinical signs include alternate bowel habits, bloating, abdominal pain, fatigue, foggy mind, limbs numbness, dermatitis, joint and muscle pain. These symptoms largely overlap with those of other wheat-related disorders, including celiac disease and wheat allergy. Given the lack of NCWS specific biomarkers, the most advanced diagnostic algorithm implies the exclusion of confounding pathologies and the assessment of symptoms during a gluten challenge.
To date we still know little about NCWS inducing factors, possible genetic predisposition and pathological mechanisms, besides the fact that they probably differ from celiac disease and wheat allergy.
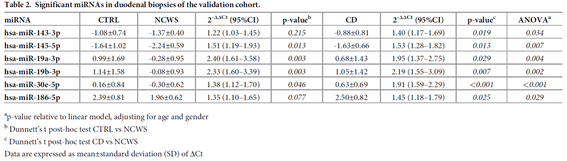
We investigated miRNA and gene expression profiles of NCWS with the aim of shedding light on the molecular mechanisms governing this pathology and eventually identifying effective biomarkers helpful to the diagnosis. In the intestinal mucosa of patients affected by NCWS we identified a few miRNAs whose expression is higher in comparison to control patients affected by gluten-independent dyspeptic symptoms and celiac disease (Table 2).
Furthermore, the intestinal mucosa of NCWS patients present differentially expressed transcripts in comparison to dyspeptic controls (Fig. 1).
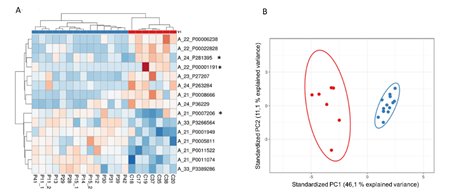
Heat map showing upregulated (red) and downregulated (blue) transcripts in controls and NCWS patients. The Scatter plot shows principal component analysis summarising the expression levels of patients’ transcripts. Red and blue dots represents controls and NCWS patients respectively
Our study provided the first evidence that NCWS patients can be genetically defined. The relevance of our finding is underscored by the current lack of biomarkers and laboratory tests useful to validate the diagnosis of NCWS. Differentially expressed genes and/or miRNA patterns combined with serological and histological exclusion of celiac disease, might represent a future marker for positive diagnosis of this syndrome. However, validation on larger groups of patients is needed to introduce this type of biomarker into the clinic.
Study of beneficial effects of natural extracts from olive leaves on human cells.
The beneficial effects of Mediterranean Nutrition on human health have been extensively documented especially with regard to the prevention of degenerative diseases such as cardiovascular diseases and cancer. This protective effect has been partly attributed to "minor components" (e.g. polyphenolic substances) present in some basic foods characteristics of the Mediterranean Nutrition. For example, extra-virgin olive oil is particularly rich in polyphenols such as oleuropein and hydroxytyrosol. Another source of antioxidants that has not so far been fully used are olive leaves. In fact, they also are particularly rich in polyphenolic substances with antioxidant activity.
We aim at characterising the amount of polyphenols and the antioxidant activity of olive leaf extract from the two main local cultivars Peranzana e Gentile. Furthermore, we will investigate the effects of such extracts on the growth of melanoma cancer cells and on cellular models of neurodegeneration.