Newborn Screening Laboratory
ABRUZZO NEWBORN SCREENING LABORATORY
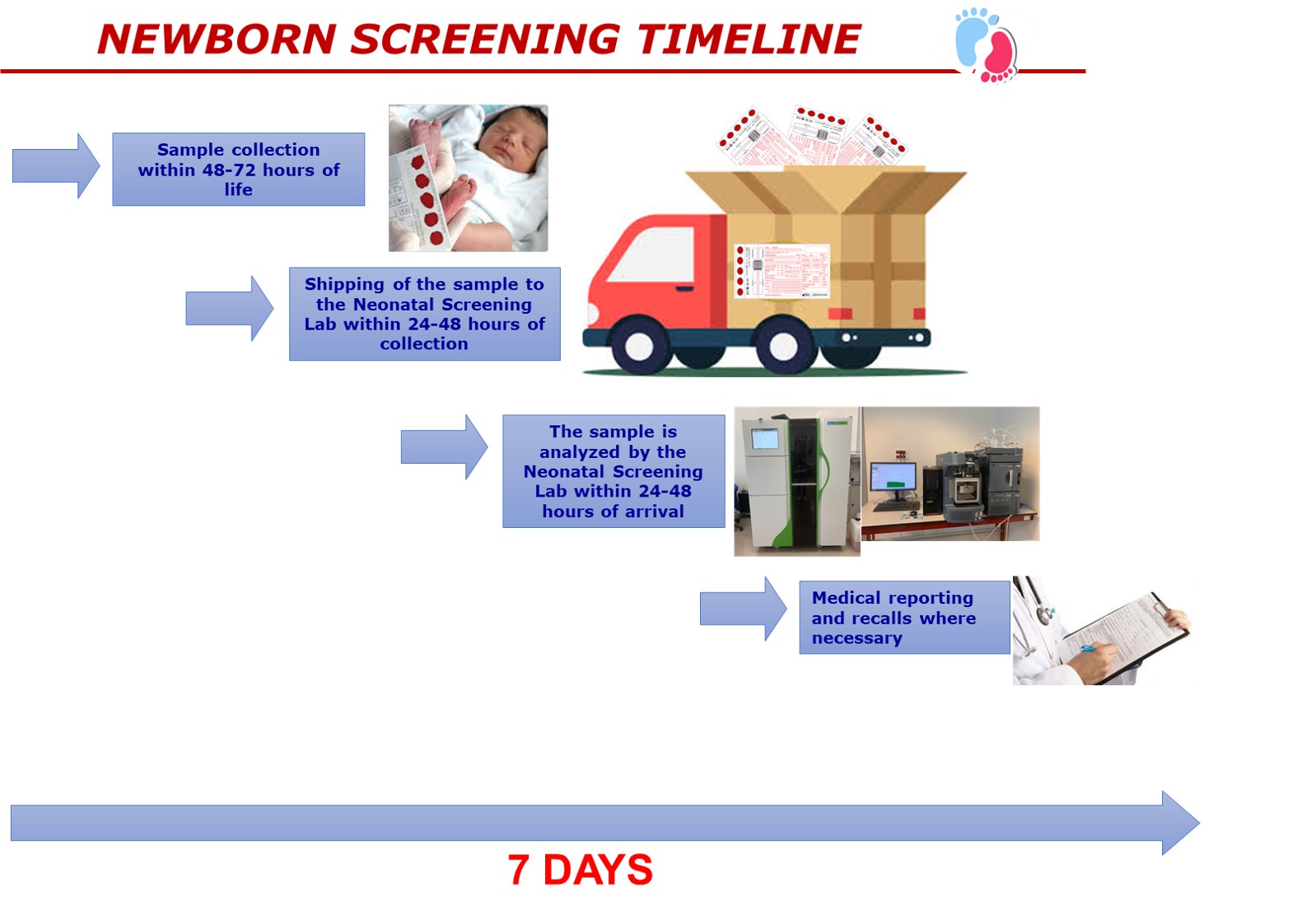

The newborn screening laboratory works in support of the Italian Newborn Screening Program (Legge 167/2016; DM 13/10/2016). Laboratory testing aims at the early detection of biochemical markers that reveal hidden congenital (present at birth) disorders. Every newborn in Abruzzo is tested for over 40 rare genetic or inherited disorders (see panel below). The laboratory processes specimens (blood spots) from over 10,000 newborns each year.
Given that time is critical in newborn screening, we receive the samples, analyze and report them within the first week of the newborn's life.

Ongoing projects and collaborations
The newborn screening laboratory works in close collaboration with the birth centers of the “Regione Abruzzo”, with the Department of Pediatrics of the Spirito Santo Hospital in Pescara, as coordination center, and with the Metabolic Diseases Unit of the Bambino Gesù Children Hospital as the diagnostic confirmation center.
Furthermore, we carry out scientific programs aimed at expanding the
biochemical knowledge of congenital errors also through the
improvement of the technological platforms used for diagnostics.
Currently, we are collaborating with Waters (Waters Corporation,
Stamford Avenue, Altrincham Road, SK9 4AX Wilmslow, UK) for the
evaluation of the novel Renata DX screening system technology.
Newborn screening panel
Amino Acid Disorders (Tandem Mass Spectrometry methodology)
- Argininemia (ARG)
- Citrullinemia I and II (CIT I; CIT II)
- Argininosuccinate Lyase Deficiency (ASA)
- Homocystinuria (MTHFR)
- Hypermethioninemia (CBS)
- Phenylketonuria (PKU)
- Benign Hyperphenylalaninemia (HPA)
- Biopterin synthesis defect (BIOPT BS)
- Biopterin Defect in Cofactor Regeneration (BIOPT REG)
- Tyrosinemia I and II (TYR I; TYR II)
- Maple Syrup Urine Disease (MSUD)
Biotinidase Deficiency (immunofluorometric methodology)
Cystic Fibrosis (immunofluorometric methodology)
- Elevated IRT values result in mutation testing
Galactosemia (immunofluorometric methodology)
- Elevated total Galactose values result in Galactose-1-Posphate Uridylyl-transferase testing
Congenital Hypothyroidism (immunofluorometric methodology)
Fatty Acid Oxidation Disorders (Tandem Mass Spectrometry methodology)
- Carnitine Uptake Deficiency (CUD)
- Carnitine Palmitoyl Transferase Deficiency (CPT I; CPT II)
- Short Chain AcylCoA Dehydrogenase Deficiency (SCAD)
- Glutaric Acidemia Type II (GA II/MADD)
- Medium Chain AcylCoA Dehydrogenase Deficiency (MCAD)
- Very Long Chain AcylCoA Dehydrogenase Deficiency (VLCAD)
- Long Chain Hydroxyl AcylCoA Dehydrogenase Deficiency (LCHAD; MCHAD; SCHAD)
- Carnitine /Acylcarnitine Translocase Deficiency (CACT)
- Trifunctional Protein Deficiency (TFP)
Organic Acid Disorders (Tandem Mass Spectrometry methodology)
- Glutaric Acidemia Type I (GA I)
- Isovaleric Acidemia (IVA)
- Beta-ketothiolase Deficency (BKT)
- 3 Hydroxy 3 Methylglutaryl CoA Lyase Deficiency (HMG)
- Propionic Acidemia (PA)
- Methylmalonic Acidemia (MUT; CbI A; CbI B; CbI C; CbI D)
- Isobutyryl CoA Dehydrogenase Deficiency (2 MBG)
- Malonic Aciduria (MAL)
- Multiple CoA Carboxylase Deficiency (MCD)
Differential Diagnosis Disorders (Tandem Mass Spectrometry methodology)
- Type III Tyrosinemia (Tyr III)
- Glycine N-methyltransferase deficiency (GNMT)
- Methionine adenosyltransferase deficiency (MAT)
- S-adenosylhomocysteine Hydrolase Deficiency (SAHH)
- 3 Methylglutaconyl CoA Hydratase Deficiency (3 MGCA)
- 3 Methyl Crotonyl CoA Carboxylase Deficiency (3 MCC)
- 3-hydroxy-2-methylbutyryl-CoA dehydrogenase deficiency (2M3HBA)
- Isobutyryl CoA Dehydrogenase Deficiency (IBG)

Equipment
- Mass spectrometer Waters UPLC –Class/Xevo TQD
- Mass spectrometer Waters Renata DX/Xevo TQD
- GSP® Newborn screening system PerkinElmer
- Panthera-Puncher™9 instrument PerkinElmer
- DBS-Puncher instrument PerkinElmer
- Victor ™ 2D instrument PerkinElmer

Mass spectrometer Waters UPLC –Class/Xevo TQD + Mass spectrometer Waters Renata DX/Xevo TQD
|
|
||
 Luca FedericiFull Professor of Biochemistry |
||
 Dr. Ilaria Cicalini, PhDShotgun proteomics; Bioinformatics; |
||
 Dr. Ada Giovanna Consalvo, PhDTargeted metabolomics; |







