Thrombosis and Haemostasis
The group has a long-term expertise in the pathophysiology of atherothrombosis, namely the role of oxidative stress and platelet activation, in the context of metabolic diseases, including diabetes and obesity. The scientific production encompasses pathophysiological and clinical pharmacology studies addressing interindividual variability in low-dose aspirin response, its determinants and possible therapeutic strategies. Know-how includes in vivo biochemical evaluation of thromboxane metabolite excretion as well as isoprostane formation, characterization of platelet proteins and platelet-monocyte aggregates by state-of-the-art flow-cytometry methods, and platelet and circulating microRNA (miRNA) expression. Current investigations address:
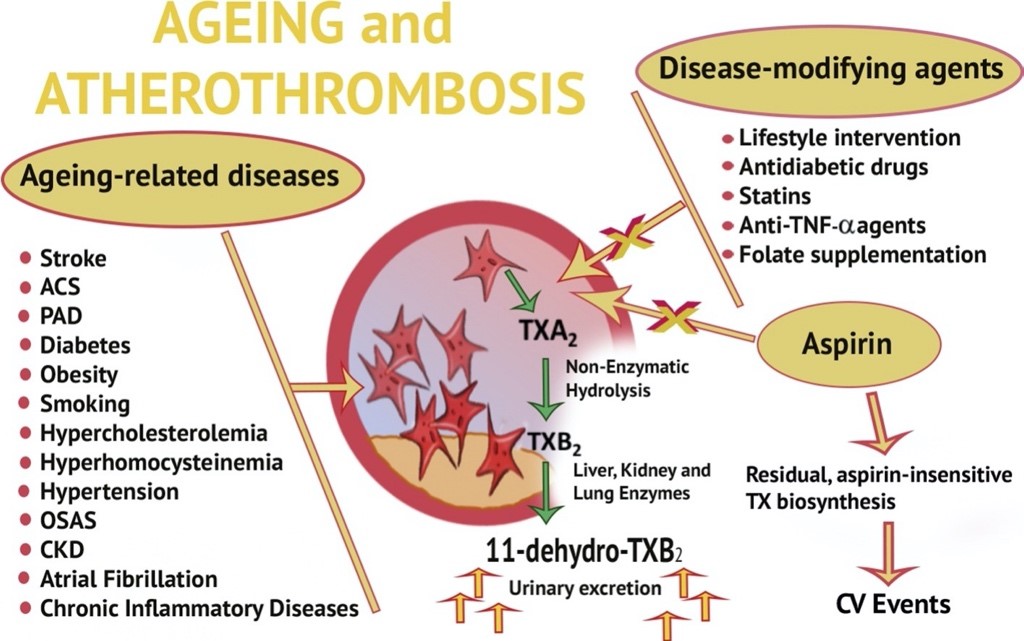
- the role of 11-dehydro-TXB2 as an index of in vivo platelet activation
Platelet activation plays a key role in atherogenesis and atherothrombosis. Non-invasive measurement of thromboxane (TX) metabolite excretion, namely 11-dehydro-TXB2 excretion, in the urines provides biochemical evidence of TX-dependent platelet activation, while avoiding artifactual platelet activation during and after blood sampling. This expertise allowed us to detect persistent TX-dependent platelet activation in a number of acute and chronic conditions associated with increased risk of atherothrombosis, including cardiovascular disease, cerebrovascular diseases, type 1 (T1DM) and type 2 (T2DM) diabetes mellitus, obesity, hypercholesterolemia, hyperhomocysteinemia, hypertension, chronic kidney disease, and chronic inflammatory diseases. Persistently increased platelet activation in vivo can be monitored through non-invasive measurement of urinary 11-dehydro-thromboxane (TX)B2 levels. This TX metabolite can be decreased by low-dose aspirin, and by several disease-modifying agents. An incomplete TX metabolite suppression predicts the future risk of cardiovascular events in aspirin-treated patients (from Simeone P, et al. Ageing Res Rev 2018; 48:51-78).READ MORE
- platelet activation and inhibition in prediabetes
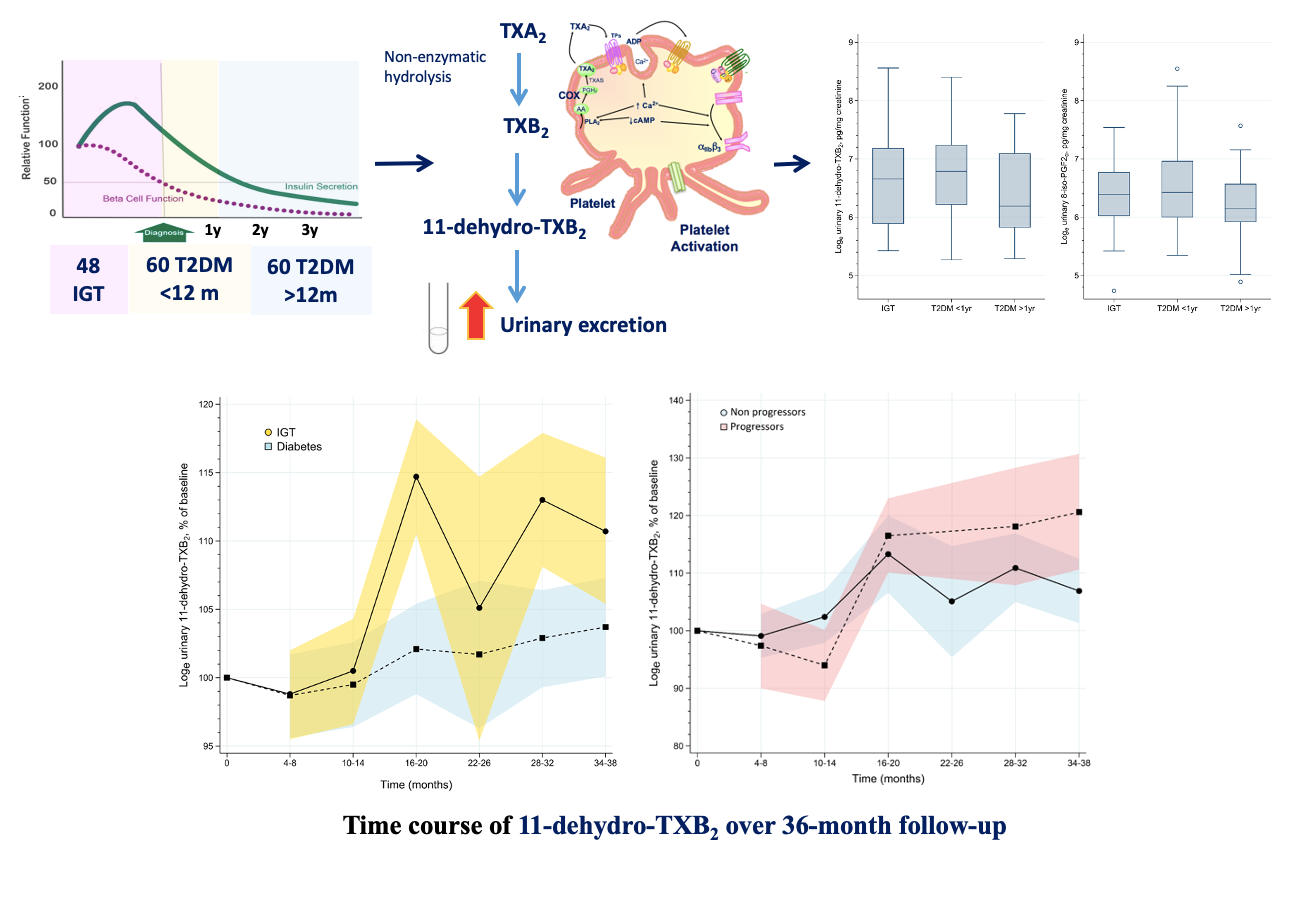
By measuring urinary 11-dH-TXB2 excretion, our group, in collaboration with other groups, has recently shown for the first time that TX-dependent platelet activation is high in subjects with a form of prediabetes, namely impaired glucose tolerance (IGT), as in patients with T2DM, and that it further increases over a 36-month median follow-up, especially in the subjects who progress to overt diabetes. These findings may provide a novel rationale for testing the efficacy and safety of antiplatelet therapy at an earlier stage of the disease than currently recommended. We also showed that in subjects with obesity and prediabetes, TX-dependent platelet activation can be mitigated by weight loss achieved with lifestyle programs or antihyperglycemic drugs with an impact on body weight, such as liraglutide. Increased TX-dependent platelet activation in subjects with IGT and enhanced 11-dehydro-TXB2 excretion in IGT patients who developed diabetes. Enhanced TXA2‐dependent platelet activation, as reflected by 11‐dehydro‐thromboxane (TX)B2 urinary excretion, is comparably abnormal in IGT as in T2DM, and it is persistent over long‐term follow‐up. Baseline TXM excretion was comparable between subjects with IGT and T2DM. During a 36‐month follow‐up, increasing urinary TXM excretion over time was observed in the subjects who progressed to diabetes vs non progressors (from Santilli F, et al. Diabetes Metab Res Rev 2020; 36:e3232).READ MORE
- inflammation, 8-iso-prostaglandin F2α formation and platelet activation
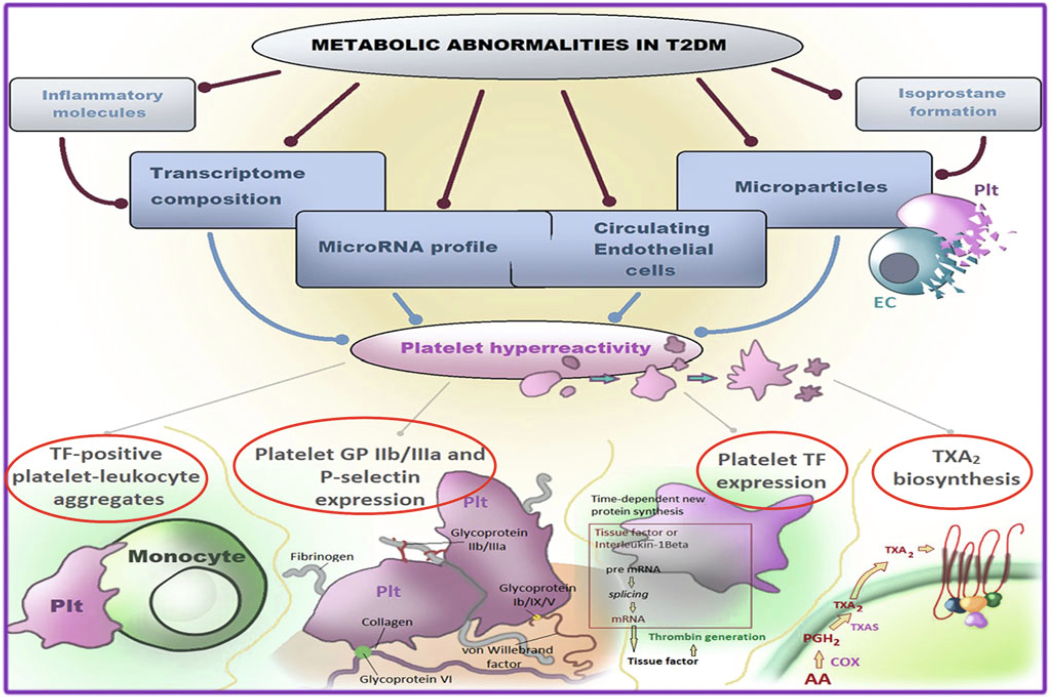
Bioactive prostaglandin (PG) F2-like compounds (isoprostanes) are produced from arachidonic acid through a nonenzymatic process of lipid peroxidation, catalyzed by oxygen free radicals on cell membranes and LDL particles. We observed a direct correlation between urinary 8-iso-PGF2α, marker of in vivo lipid peroxidation, and 11-dehydro-TXB2 excretion, supporting the hypothesis that persistent platelet activation may, at least in part, be related to enhanced formation of biologically active products of arachidonic acid peroxidation. In clinical settings characterized by accelerated atherothrombosis, we showed that the underlying metabolic abnormality (hyperglycemia, insulin resistance, hypercholesterolemia, hyperhomocysteinemia) is the primary trigger of low-grade inflammation, lipid peroxidation and TX-dependent platelet activation. In these contexts, platelets are potent triggers of inflammation, tissue damage, accelerated atherogenesis and further platelet activation, through the release of a number of proteins, miRNA, microvesicles. Our group studies the complex role of the “platelet releasate” in the pathogenesis of atherothrombosis. Metabolic abnormalities in type 2 diabetes. The metabolic abnormalities associated with T2DM may affect platelet transcriptome and/or posttranscriptional regulation through intermediate mediators, such as oxidative stress with isoprostane formation, inflammatory molecule production, endothelial dysfunction with circulating endothelial cells and microparticle release, and cross talk between cells with miRNA exchange through circulating microparticles. The variable contribution and still poorly characterized interaction among these mechanisms result in platelet hyperreactivity (from Santilli F, Simeone P, Liani R, Davì G. Platelets and Diabetes. Chapter in: Platelets in Thrombotic and Non-Thrombotic Disorders. Gresele P; 2017, pp.1225-1238).READ MORE
- anti-hyperglycemic drugs and cardio-metabolic complications
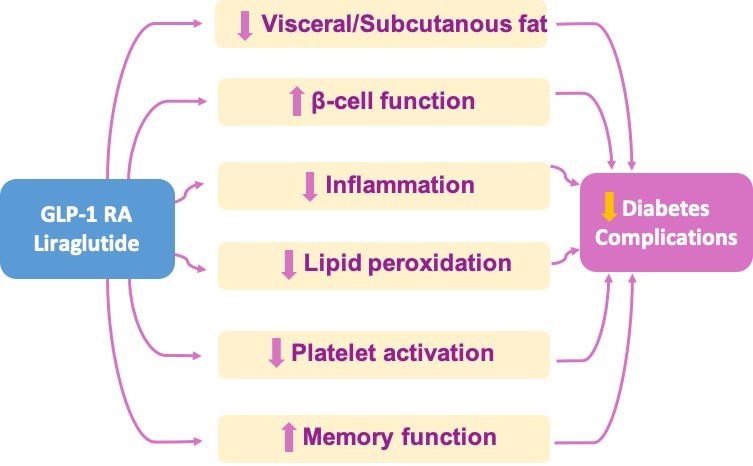
Other anti-hyperglycemic strategies are currently being tested to investigate the pathophysiological mechanisms underlying their benefits in the prevention of obesity- and diabetes-related complications. Cardiometabolic effects of liraglutide in obese subjects with prediabetes or diabetes mellitus. By performing a randomized study of liraglutide vs. lifestyle changes to achieve comparable weight loss, we unraveled that liraglutide improves visceral fat loss, β-cell function, lipid peroxidation and platelet activation, as well as memory function, in obese patients with prediabetes or early diabetes mellitus (from Vadini F, et al. Int J Obes (Lond); 2020, Jan 21. Simeone P, et al. Nutrients 2018, Dec 2;10(12). Santilli F, et al. Diabetes Care 2017;40:1556-1564).READ MORE
Obesity is associated with an increased risk of T2DM and cardiovascular complications. The risk depends significantly on adipose tissue distribution. Cardiovascular outcome trials showed that Liraglutide, a glucagon-like peptide 1 receptor agonist (GLP-1RA), is associated with reduced risk of cardiovascular events and mortality, while promoting weight loss and improving glycemic control. Our group has reported that in obese subjects with prediabetes or early T2DM randomized to liraglutide treatment or lifestyle changes to achieve comparable weight loss, a significantly enhanced abdominal visceral fat loss and improved β-cell function was obtained with liraglutide. This study showed that the liraglutide effects on visceral obesity and β-cell function may justify, at least in part, its cardiovascular benefit, and may provide a rationale for its use in obese subjects in an early phase of the natural history of glucose metabolism dysregulation.
Diabetic subjects are also at increased risk of subtle cognitive impairment since the disease early stages and of dementia later in life. In animal models, GLP1-RAs have been shown to exert neuroprotective effects, especially in the memory domain. In this regard, we showed for the first time in humans with obesity and diabetes or prediabetes, that liraglutide is able to slow down memory function decline in diabetic patients in early, and possibly preclinical stages of the disease.
- interindividual variability in the response to aspirin (ASA)
Serum TXB2 is an index ex vivo of the maximal platelet activity that reflects the capacity to generate cyclooxygenase (COX)-1-dependent TXA2. By studying the recovery rate of platelet COX-1 activity during the 12 to 24h dosing interval of aspirin administration, in aspirin-treated subjects with and without diabetes, we characterized substantial interindividual variability, and this abnormal biochemical phenotype could be completely reversed by aspirin 100 mg given twice daily. Thus, we showed that the variable turnover rate of the drug target represents the main determinant of the interindividual variability in aspirin response. Possible determinants of interindividual variability in response to aspirin. A suboptimal response to aspirin can be due to: (1) an impaired acetylation and inhibition of COX-1, mainly related to decreased absorption of the drug, and to oxidative stress-induced lipid hydroperoxide and peroxynitrite generation; (2) an increase in aspirin-insensitive agonists, related to low-grade inflammation, with increased TXA2 formation by platelet, or to oxidative stress, with enhanced TX receptor activation by 8-iso-PGF2α; and (3) an accelerated recovery of COX-1 activity linked to an enhanced platelet turnover (from Santilli F and Simeone P. Intern Emerg Med 2019;14:1217-1231).READ MORE
Along these lines, we performed a subsequent study to characterize, in diabetic vs non-diabetic patients on aspirin 100 mg od, the platelet phenotype, in terms of miRNA profile and proteome, associated with inadequate response to aspirin. We expect to identify platelet or circulating biomarkers of less-than-expected aspirin response, for disease-tailored therapeutic regimens. For this project, the PI has been awarded a Grant from the Italian Ministry of Health. (Figure)
- Altered miRnome and platelet reactivity
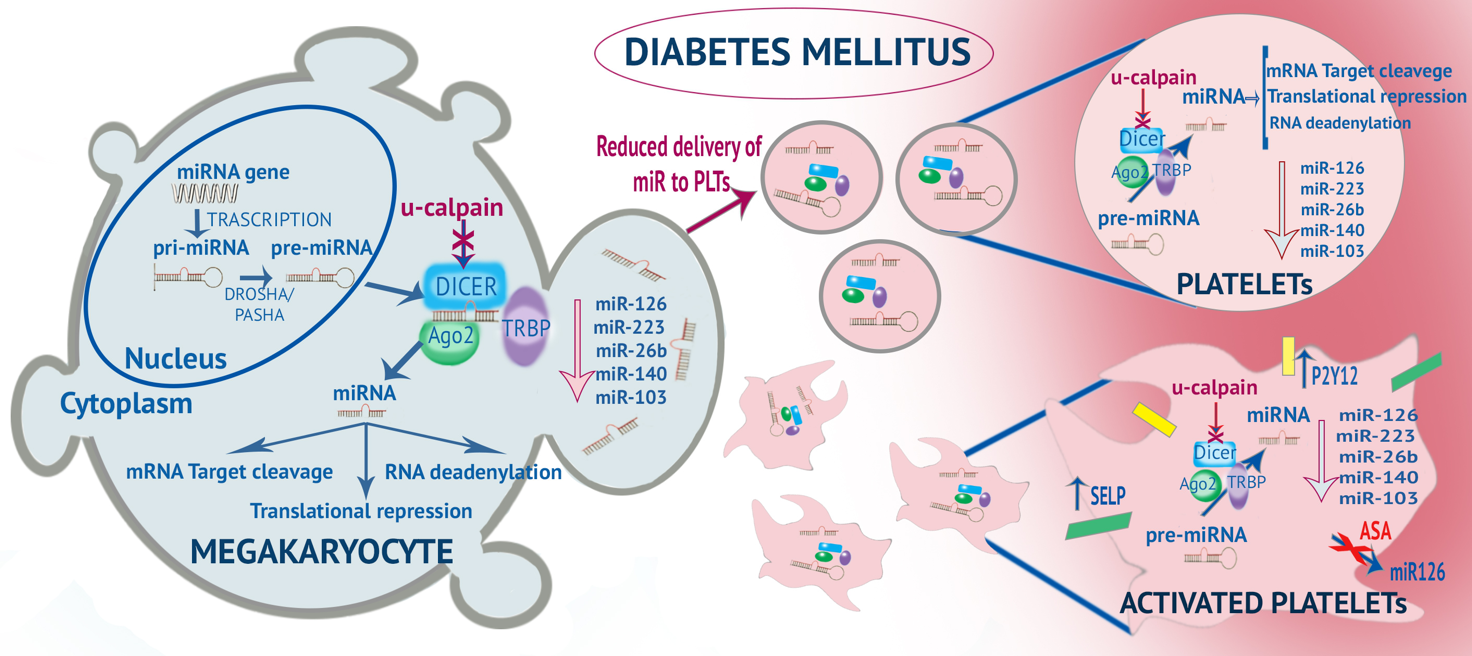
Platelets carry messenger RNAs (mRNAs), a large number of micro(mi)RNAs and several proteins involved in miRNA processing (e.g., Dicer, TRBP2, Ago2), which are delivered from MKs. Several studies have reported altered levels of miRNA in platelets from patients with DM. The levels of some miRNA (miR-223, miR-26b, miR-126, mir103, and miR-140) are significantly lower in both platelets and MKs of patients with T2DM. This is likely due to overactivation of calpain, which impairs proteolytic degradation of Dicer resulting in decreased levels of several platelet enriched miRNAs and accumulation of pre-miRNAs, and to altered capacity of MKs to deliver sufficient amount of miRNAs and their precursors into platelets. This altered miRNA profile of platelets results in enhanced platelet function/activation, since miR-223 regulates the expression of platelet P2Y12 receptor, and miR- 26b and miR-140 target the SELP mRNA that encodes P-selectin. Activated platelets transfer miR-126 from the platelet to the plasma compartment, an effect inhibited by low-dose aspirin. MiRNA formation and altered miRNA profile in diabetes. Platelet hyperreactivity in diabetes mellitus and relationship between altered miRNome in megakaryocytes and platelets (from Santilli F, Simeone P, Liani R. Platelets, Fourth Edition. Chapter 27: The Role of Platelets in Diabetes mellitus. Michelson AD. Elsevier/Academic Press; 2019, pp.469–504).READ MORE
- platelet activation and inhibition in HIV infection
READ MORE
In HIV-1-infected patients, characterized by an increased risk of ischemic cardiovascular (CV) events, the pathogenic mechanisms underlying CV risk remain elusive. Immune activation, inflammation, and persistent platelet activation are regarded as the underlying events leading to accelerated atherogenesis and thrombosis in this setting. Aspirin has been shown to attenuate platelet activation and immune responses in HIV infected patients but is under-prescribed in this setting. We are currently investigating the role of platelet activation and inhibition in HIV-infected patients, and whether an interindividual variability in the response to low-dose aspirin may exist, possibly yielding a less-than-expected response to aspirin at least in a fraction of HIV-infected patients.
- JAK inhibitors and platelet activation in patients with rheumatoid arthritis
READ MORE
Atherothrombosis is a common comorbidity associated with rheumatoid arthritis (RA) and inflammation. Cytokine signaling via JAK pathways leads to further induction of inflammatory gene expression, which continues the loop of inflammatory signaling. Persistent platelet activation/hyperreactivity, with enhanced TX biosynthesis, has a major role in the extent of systemic inflammation and lipid peroxidation in RA. In particular, JAK-2-dependent signaling may affect thrombopoiesis, as well as platelet activation. Thus, drugs targeting JAK-2, such as tofacitinib, have the potential to contrast reactive thrombocytosis, and negatively modulate platelet hyperreactivity.
For this reason, our group designed a study aimed at exploring the role of platelets in autoimmunity, supporting the possibility that platelets are an attractive target in RA. Our goal is to assess, in patients with RA, the effects of 6-month tofacitinib treatment on markers of platelet activation and platelet turnover/regeneration, and to correlate the change in markers of platelet activation/turnover/regeneration with change in systemic inflammation, and disease progression. Funding: INNOVARE 2018 (INflammation and ImmuNology COmpetitiVe GrAnt ProgRam for REsearch)
Santilli F, Zaccardi F, Liani R, Petrucci G, Simeone P, Pitocco D, Tripaldi R, Rizzi A, Formoso G, Pontecorvi A, Angelucci E, Pagliaccia F, Golato M, De Leva F, Vitacolonna E, Rocca B, Consoli A, Patrono C. In Vivo Thromboxane-Dependent Platelet Activation Is Persistently Enhanced in Subjects With Impaired Glucose Tolerance. Diabetes Metab Res Rev 2020; 36(2):e3232. PMID: 31671234. https://doi.org/10.1002/dmrr.3232
Vadini F, Simeone P, Boccatonda A, Guagnano MT, Liani R, Tripaldi R, Di Castelnuovo A, Cipollone F, Consoli A, Santilli F. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study. Int J Obes (Lond) 2020; Jan 21. PMID: 31965072. https://doi.org/10.1038/s41366-020-0535-5
Santilli F and Simeone P. Aspirin in primary prevention: the triumph of clinical judgement over complex equations. Intern Emerg Med 2019; 14(8):1217-1231. https://doi.org/10.1007/s11739-019-02191-4
Santilli F, Simeone P, Liani R. Platelets, Fourth Edition. Chapter 27: The Role of Platelets in Diabetes mellitus. Edited by Alan D. Michelson. Elsevier/Academic Press 2019.
Simeone P, Liani R, Tripaldi R, Di Castelnuovo A, Guagnano MT, Tartaro A, Bonadonna RC, Federico V, Cipollone F, Consoli A, Santilli F. Thromboxane-Dependent Platelet Activation in Obese Subjects with Prediabetes or Early Type 2 Diabetes: Effects of Liraglutide- or Lifestyle Changes-Induced Weight Loss. Nutrients 2018; Dec 2;10(12). PMID: 30513818 PMCID: PMC6315606; https://doi.org/10.3390/nu10121872
Patti G, Cavallari I, Andreotti F, Calabrò P, Cirillo P, Denas G, Galli M, Golia E, Maddaloni E, Marcucci R, Parato M, Pengo V, Prisco D, Ricottini E, Renda G, Santilli F, Simeone P, De Caterina R. Prevention of atherothrombotic events in patients with diabetes mellitus: from antithrombotic therapies to new-generation glucose-lowering drugs. Nat Rev Cardiol 2018; Sep 24. https://doi.org/10.1038/s41569-018-0080-2
Simeone P, Boccatonda A, Liani R, Santilli F. Significance of urinary 11-dehydro-thromboxane B2 in age-related diseases: Focus on atherothrombosis. Ageing Res Rev 2018; Dec;48:51-78. Review. PMID: 30273676; https://doi.org/10.1016/j.arr.2018.09.004
Santilli F, Simeone PG, Guagnano MT, Leo M, Maccarone MT, Di Castelnuovo A, Sborgia C, Bonadonna RC, Angelucci E, Federico V, Cianfarani S, Manzoli L, Davì G, Tartaro A, Consoli A. Effects of Liraglutide on Weight Loss, Fat Distribution, and β-Cell Function in Obese Subjects With Prediabetes or Early Type 2 Diabetes. Diabetes Care 2017; 40(11):1556-1564. https://doi.org/10.2337/dc17-0589
Halvorsen B, Santilli F, Scholz H, Sahraoui A, Gulseth HL, Wium C, Lattanzio S, Formoso G, Di Fulvio P, Otterdal K, Retterstøl K, Holven KB, Gregersen I, Stavik B, Bjerkeli V, Michelsen AE, Ueland T, Liani R, Davi G, Aukrust P. LIGHT/TNFSF14 is increased in patients with type 2 diabetes mellitus and promotes islet cell dysfunction and endothelial cell inflammation in vitro. Diabetologia 2016; 59:2134-44. https://link.springer.com/article/10.1007/s00125-016-4036-y
Santilli F, Liani R, Di Fulvio P, Formoso G, Simeone P, Tripaldi R, Ueland T, Aukrust P, Davì G. Increased circulating resistin is associated with insulin resistance, oxidative stress and platelet activation in type 2 diabetes mellitus. Thromb Haemost 2016; 116(6):1089-1099. PMID: 27709225; https://www.thieme-connect.com/products/ejournals/abstract/10.1160/TH16-06-0471
Di Sabatino A, Santilli F, Guerci M, Simeone P, Ardizzone S, Massari A, Giuffrida P, Tripaldi R, Malara A, Liani R, Gurini E, Aronico N, Balduini A, Corazza GR, Davì G. Oxidative stress and thromboxane-dependent platelet activation in inflammatory bowel disease: effects of anti-TNF-α treatment. Thromb Haemost 2016; 116(03):486-95. PMID: 27305860; https://www.thieme-connect.com/products/ejournals/abstract/10.1160/TH16-02-0167
Santilli F, Marchisio M, Lanuti P, Boccatonda A, Miscia S, Davì G. Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb Haemost 2016; 1;116(02):220-34. PMID: 27173919; https://www.thieme-connect.com/products/ejournals/abstract/10.1160/TH16-03-0176
Santilli F Lapenna D, La Barba S, Davì G. Oxidative-stress related mechanisms affecting response to aspirin in diabetes. Free Rad Biol Med 2015; 80:101-110. PMID: 25530150; https://doi.org/10.1016/j.freeradbiomed.2014.12.010
Lattanzio S, Santilli F, Liani R, Vazzana N, Ueland T, Di Fulvio P, Formoso G, Consoli A, Aukrust P, Davì G. Circulating Dickkopf-1 in diabetes mellitus: association with platelet activation. Effects of improved metabolic control and low-dose aspirin. J Am Heart Assoc 2014; 3(4). pii: e001000. https://doi.org/10.1161/JAHA.114.001000
Santilli F, Vazzana N, Iodice P, Lattanzio S, Liani R, Bellomo RG, Lessiani G, Perego F, Saggini R, Davì G. Effects of high-amount-high-intensity exercise on in vivo platelet activation: Modulation by lipid peroxidation and AGE/RAGE axis. Thromb Haemost 2013; 110(06):1232-40. PMID: 24030807; https://www.thieme-connect.com/products/ejournals/abstract/10.1160/TH13-04-0295
Rocca B, Santilli F, Pitocco D, Mucci L, Petrucci G, Vitacolonna E, Lattanzio S, Mattoscio D, Zaccardi F, Liani R, Vazzana N, Del Ponte A, Ferrante E, Martini F, Cardillo C, Morosetti R, Mirabella M, Ghirlanda G, Davì G, Patrono C. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost 2012; 10:1220-30. PMID: 22471290; https://doi.org/10.1111/j.1538-7836.2012.04723.x
Santilli F, Vazzana N, Liani R, Guagnano MT, Davì G. Platelet activation in obesity and metabolic syndrome. Obes Rev 2012; 13:27-42. Review. PMID: 21917110; https://doi.org/10.1111/j.1467-789X.2011.00930.x
Santilli F, Rocca B, De Cristofaro R, Lattanzio S, Pietrangelo L, Habib A, Pettinella C, Recchiuti A, Ferrante E, Ciabattoni G, Davì G, Patrono C. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: implications for aspirin "resistance". J Am Coll Cardiol 2009; 53(08):667-77. https://doi.org/10.1016/j.jacc.2008.10.047
Santilli F, Davì G, Consoli A, Cipollone F, Mezzetti A, Falco A, Taraborelli T, Devangelio E, Ciabattoni G, Basili S, Patrono C. Thromboxane-dependent CD40 ligand release in type 2 diabetes mellitus. J Am Coll Cardiol 2006; 47(02):391-7. https://doi.org/10.1016/j.jacc.2005.03.079
Basili S, Ciabattoni G, Patrono C. Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus: role of interleukin-6 and disease duration. Circulation 2003; 107:3199-203. https://doi.org/10.1161/01.CIR.0000074205.17807.D0




